Atoms and Molecules
Atoms and Molecules is the basic for class 9 which cover the syllabus of Science which consist the law of fundamental chemistry, Dalton atomic law, and basic idea of atom, molecules and mole study with numerical example.
Atoms and Molecules can be understood by learning law of Chemical combination with some examples
LAW OF CHEMICAL COMBINATION :
The chemical reaction between two or more substances giving rise to products is governed by certain laws. These laws are called laws of chemical reaction ,
They are
a) law of conservation of mass
b) Law of constant proportion
c)Law of multiple proportion Not in syllabus
d)Law of reciprocal proportion ”
e) Gay Lussac’s law of combing volume ”
LAWOF CONSERVATION OF MASS in Atoms and Molecules:
Mass can neither be created nor can be destroyed in yht chemical reaction this law can be understood ” During the chemical reaction total mass of reactant will be equal to the total mass of the product ”
A+ B ——-> AB ie mass of A + mass of B = mass of AB
2 Na + Cl2 ——-> 2 NaCl ( Mass of Na + Mass of Cl = Mass of NaCl) let us suppose 5gm of Na react with 6gm of Cl it gives 11gm of NaCl .
LAW OF CONSTANT PROPORTION in Atoms and Molecules :
A pure chemical compound always contain the same element combine together in same proportion by mass .
Example 18 gm of H2O = 16 gm of O2 + 2 gm of H2 ratio of mass of H2 ; mass of O2 = 2:16=1:8 by mass ,if oxygen is 32 gm than it requires 4 gm of hydrogen to form 36 gm of water.
DALTONS ATOMIC THEORY : Itis based on the laws of chemical combination, It provides the explanation for law of conservation of mass and law of constant composition The postulates of the theory are
a) All matter is made up of very small tiny particles called atoms
b) Atoms are indivisible particles which can neither be created nor can be destroyed in chemical reaction (law of conservation of mass)
c) Atoms of the element have identical mass and chemical properties
d) Atoms of different elements have different mass and properties
e)Atoms combine in the ratio of small whole number to form compound (Law of constant proportion )
f) Relative number and kinds of atoms are constant in given compound .
ATOM ;
Itis the smallest particle of an element which takes part in chemical reaction such that it maintain it’s identity through the chemical or physical changes . They are so small that cannot be seen through naked eye/ powerful microscope. Radius of smallest atom hydrogen is .037 nm
ATOMIC MASS :
The mass of the atom of an element is called its atomic mass. It is defined as atomic mass unit which is defined as the quantity of mass equal to an atom of mass of carbon -12 1amu = 1/12 mass of carbon atom C-12
1amu = 1.66 x10-27 kg
Element name/ symbol / atomic mass / valency :
i) Hydrogen/ H / 1/+ 1
ii) Helium /He /4 / zero
iii) Lithium / Li/ 7/+1
iv) Beryllium/ Be/9/+2
v)Boron/B/11/+3
vi)carbon/C/12/+4
vii) Nitrogen/N/ 14/ -3
viii) oxygen / O/16/ -2
ix) Fluorine /F/19 /-1
x) Neon/Ne/2o/ zero.
xi) sodium / Na/ 23/+1
xii) Magnesium/Mg/ 24/ +2
xiii) Aluminium/Al/27/+3
xiv) Silicon/Si/28/ +4
xv) Phosphorous/P/ 31/+5 or -3
xvi) Sulphur/S/32 /-2
xvii) Chlorine /Cl/35/ -1
xviii) Potassium/K/39/+1
xix) Calcium /Ca/ 40/ +2
xx) Iron /Fe/ 56/ +3
HOW DO ATOM EXIST :
Atom of most of the elements are very reactive and does not exist in free state only the atoms of noble gases ( He, Ne, Ar , Kr ,Xe and Rn) are chemically inactive therefore exists in free state as monotonic atoms of all other elements combine together to form molecules or ions .
Mostly atom are electrically neutral in which number of electron is equal to number of proton . It transfer of electron takes place it form the ions when atom loses the electron it is +ive charge known as cation and if atom gains the electrons becomes -ive charge called anions.
MOLECULE :
A molecule is group of two or more atoms which are chemically bonded with each other ,it is the smallest particles of matter ( expect element ) which is capable of an independent existence and show all properties of substance eg H,O is the smallest particle of water which show all the properties of water ,
A molecules may have atom of same /different elements depending upon this , molecule can be categorized into two categories:
Homoatomic molecule (having atoms of same element ) H2, O2,N2, S8
Heteroatomic molecules compounds ( compounds contain different atoms ) H2O, NH3, CO2.
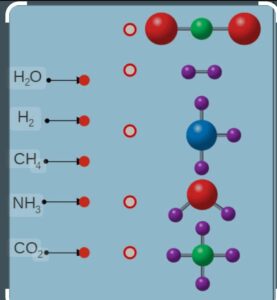
ATOMICITY :
The number of atoms present in one molecule of an element is called its atomicity .
Monoatomic have single atoms : He, Ar , Ne
Diatomic having two atom O2 , H2 ,
Polyatomic : P4 , O3
CHEMICAL FORMULA :
It is a symbolic representation of the composition of compounds.
To form the chemical formula the valencies/charge on ions must be balance when compound is formed metal and non metal comes first as Cao .
when poly atomic ions are formed are used , the ions are enclosed writing the number to show the ratio Ca (OH)2.
MOLECULAR MASS :
It is the sum of atomic masses of all atoms in one mole example H2O molecular mass = 2x atomic mass of hydrogen + atomic mass of oxygen =2×1 + 16 = 2+16= 18u
FORMULA MASS UNIT :
It is the sum of atomic masses of ions and atoms present in formula for a compound
. NaCl = Atomic mass of Na + atomic mass of chlorine = 23 + 35.5= 58.5
formation of formula :
i) Hydrogen sulphide H : S = Valency 1;2 = formula H2S
ii) for carbon di oxide C;O= valency 4; 2 =valency 2:1 = formula CO2
iii) for Hydrochloric acid H: Cl= valency 1:1 = formula HCl
iv) for carbon tetra chloride C;Cl = valency 4:1 = formula CCl4
v) For magnesium chloride Mg: Cl =valency 2:1 = formula Mg Cl2
IONS :
An ion may be defined as an atom or group of atoms having positive or negative charge
positive charge = Na^+, K^+ , Ca^2+ negative charge Cl^- , S^2- , SO4^2-
Chemical formula :Na : CO3 = valency 1 :-2= formula Na2CO3 (Sodium carbonate)
Aluminium Sulphate = Al: SO4 = valency 3 : -2 = Al2(SO4)3
MOLAR MASS :
The molar mass of substance is the mass of one mole of substance it is equal to 6.023×10^23 atoms of that elements.
Atomic mass of hydrogen is 1 u which is equal to molar mass 1g/mol in thxe same way molar mass of nitrogen atom is 14gm /mol ,molar mass of S8 is 32×8 =256gm/mol
MOLE CONCEPT :
A group of atoms/ molecule/ions contain 6.023 x10^23 particles in one mole of that substances.
1 mole atom =6.022x 10^23 atoms/ 1 mole of molecules = 6.022×10^23 molecules
6.022×10^23 is called Avogadro number ,one mole of substance has mass equal to gram atomic mass of the element .
IMPORTANT FORMULAE
a) number of mole = mass of substance / molar mass of the substance= m/M b) number of moles = given number of particles/ avogadro’s number
example 1 : calculate no of iron atoms in piece of iron of 2.8 gm
first calculate no. of moles =mass of substance/ molar mass of substance= 2.8/56=1/20=.05
for calculating no. of atom = no of moles x Avogadro’ s number =.05×6.022×10^23=3.011×10^23
example 2 : Mass of one molecule of a substance is 5.33×10^-23 than for molar mass multiply the mass of one molecule to Avogadro’s number ie 5.33×10^-23 x6.022×10^23= 32gm/mol
Conclusion : Atoms and Molecules is the basics chapter that covers the basic idea of atom and molecules with respect to numerical basis, valency , making of formula in chemistry .
Conclusion :Atoms and Molecules
In chapter Atom and Molecules basic idea of law of chemical combination, Daltons law, basic definition of atom, molecule, molar mass, mole concept ,chemical formulae basic is given according to syllabus of class 9 th to show the right path to the students.
Follow us on: Facebook
Read More: Structure of Atom