Structure of Atom
Structure of Atom is the chapter from chemistry that covers the syllabus of class 9 th which gives basic idea of atom.
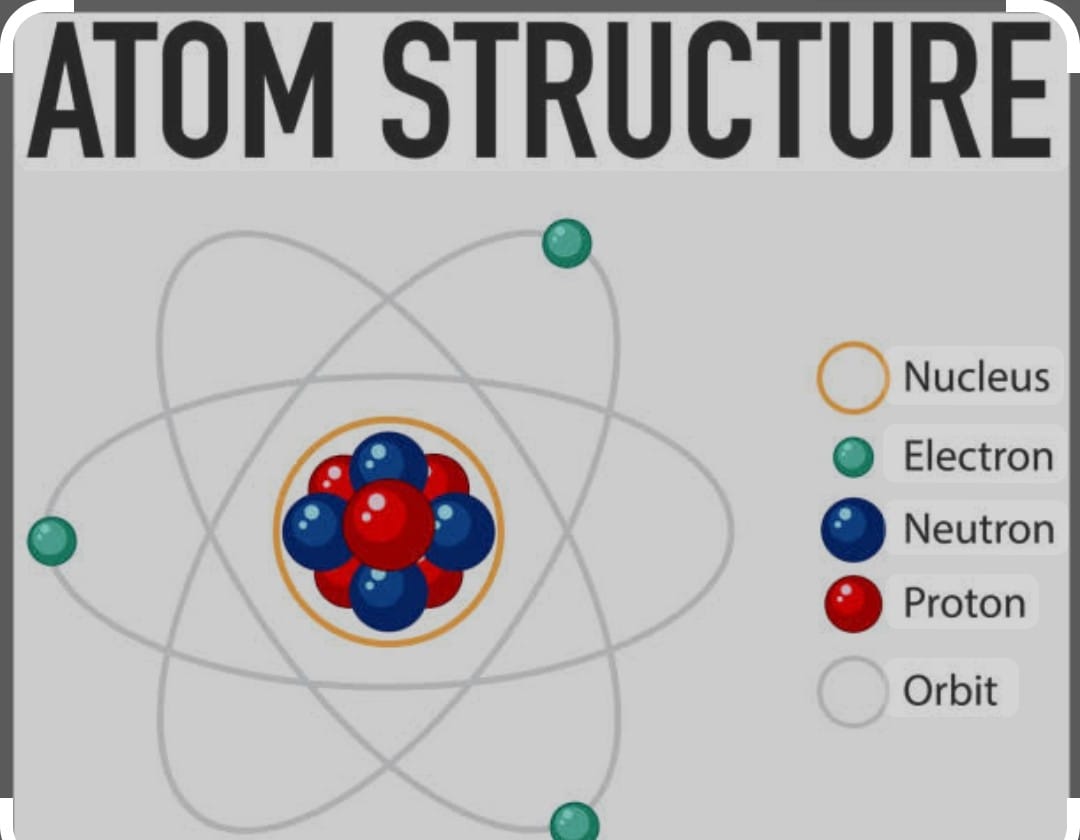
Background of Discovery of Atom
Jhon Dalton considered atoms an indivisible entity , but his concept was discarded in 19th century when scientist were able to find existence of charge particles as electron and proton and neutral particle as neutrons in atoms These particles are known as sub- atomic particles.
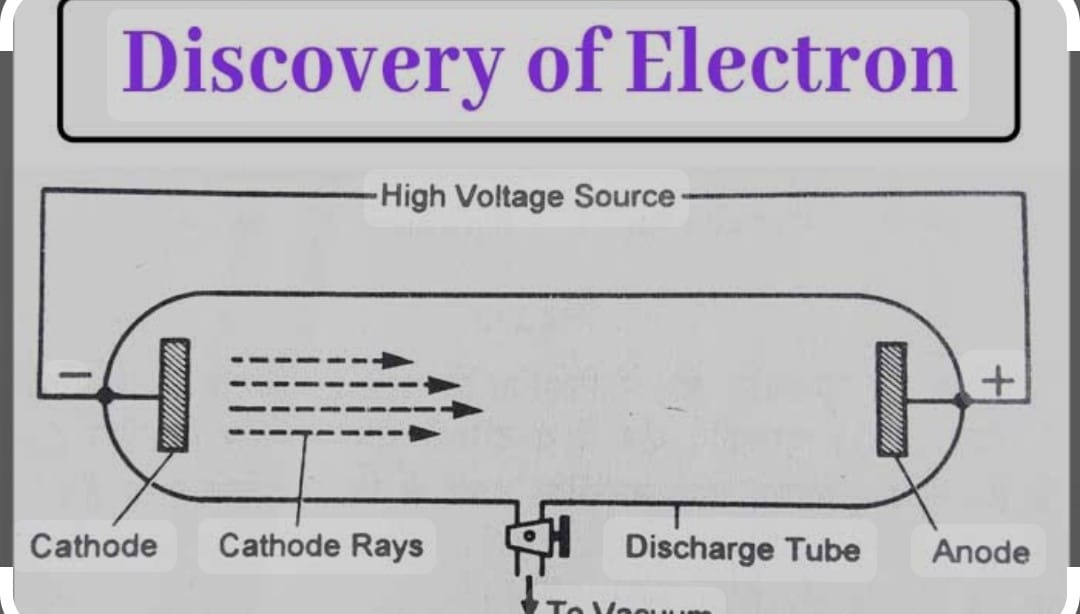
DISCOVERY OF ELECTRONS ( CATHODE RAYS ) BY J.J. THOMSON.
He explained the presence of electrons during the study of cathode rays .Cathode Rays:
1. Negatively charged particles (electrons)
2. Travel from cathode to anode in a discharge tube
3. Deflected by electric and magnetic fields
4. Can produce fluorescence on impact
5. Can penetrate thin metal foils
6. Travel in straight lines
7. Carry kinetic energy and momentum
The facts charge on electron is – 1.6x 10^-19 coulomb, mass of electron was calculated by Robrt E Millikan as 9.1 x10^-27 kg
DISCOVERY OF PROTONS is done by E Goldstein by his famous anode rays/ canal rays experiment was able to detect that Canal Rays:
1. Positively charged particles (usually ionized gas atoms)
2. Travel from anode to cathode in a discharge tube
3. Deflected by electric and magnetic fields (opposite direction to cathode rays)
4. Less penetrating than cathode rays
5. Heavier and slower than cathode rays
6. Mass depends on the gas in the discharge tube
7. Can also cause fluorescence, but less intense than cathode rays the presence of positively charged particles called protons in the atom it’s charge is + 1.6×10^-19 coulomb mass is 1.673x 10^-27kg of 184times of mass of electrons
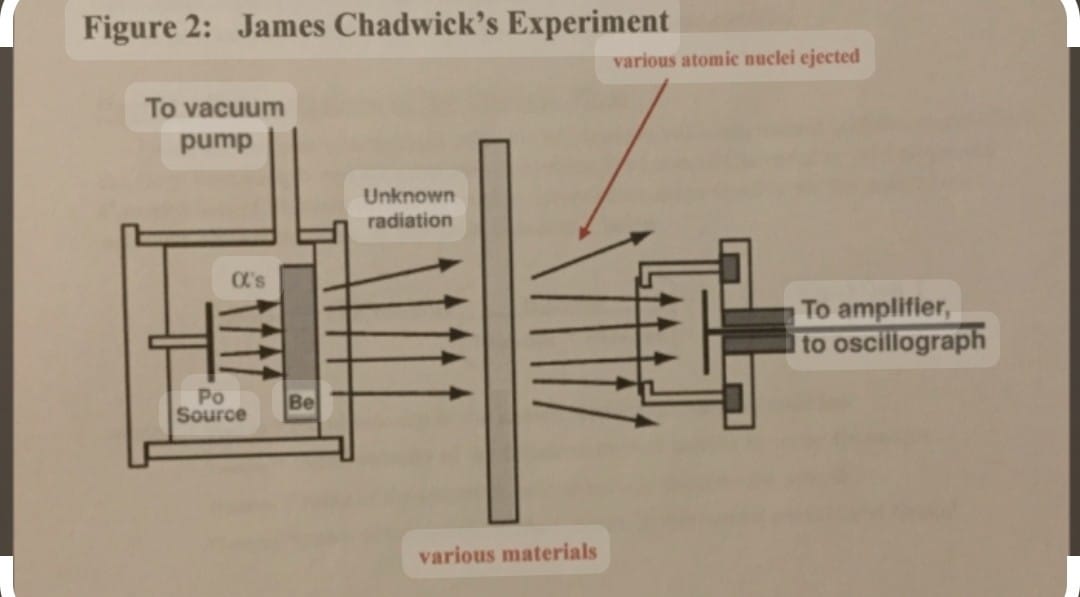
DISCOVERY OF NEUTRONS is done by J. CHADWICK
when he bombarded lighter elements like lithium/ boron with alpha particles and observed emission of new particles having zero charge but mass equal to that of proton. These particles were came to be known as neutron.
Neutron are absent in protium ( isotopes of hydrogen ) 1H1 as the mass of electron is negligible as compared to proton / neutron hence sum of mass of proton and mass of neutron will give us atomic mass
ATOMIC MASS= MASS OF PROTON + MASS OF NEUTRON
ATOMIC MODELS :
by the knowledge of presence of sub atomic particles that is electron, proton and neutron various atomic models were proposed by different scientists such as THOMSON ATOMIC MODEL , RUTHERFORDS MODEL OF ATOM , BOHR’S ATOMIC MODEL The most trusted and scientifically established model of atom which is adopted was QUANTUM MECHANICAL MODEL OF ATOM
THOMOSON’S ATOMIC MODEL :
It is also called Water Melon Model . He predicted the presence of electrons inside the positive sphere ( made of proton )just as seeds of watermelon are embedded in the edible part of watermelon .
Although this model explained neutrality of atom but couldn’t able to explain other scientific experiments conducted on atom . but was discarded .
RUTHERFORD’S ATOMIC MODEL :
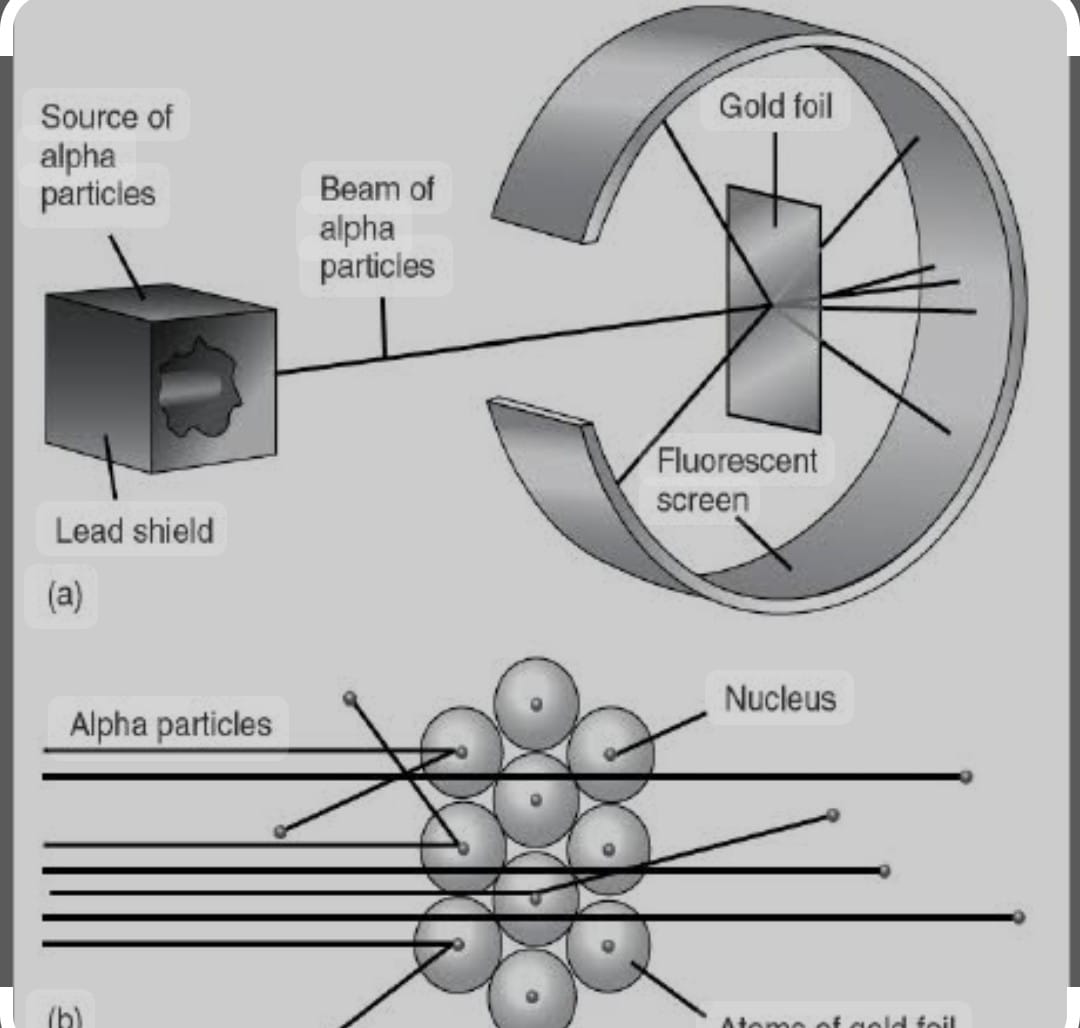
It is famous alpha rays scattering experiment Rutherford bombarded alpha rays upon thin gold foil .He made the following observation from the experiment that
i) Most of alpha particles passed through the gold foil undeflected
ii) Some of the particles deflected by foil by small angles
iii) One out of 10000 particles retraces it’s path
Rutherford Conclusions : Atom consist of predominantly empty space as most of the alpha particles gold foil undeflected . Atom contains centrally placed positively charged the nucleus carrying the +ively charged particles because few alpha particles wee deflected and bounced back .
Since minute fraction of alpha particles suffered deflection and very few retraces it’s path this lead to conclusion that most of the space an atom is empty and the space is occupied by nucleus is negligible compared to the empty space
The size of nucleus was about 10^-5 times that of size of the atom. Whole of the atomic mass is concentrated in the nucleus.
On the basis of the experiment Rutheford proposed model of the atom having following features :There is positively charged particles in the nucleus of the atom Nearly all the mass resides in the nucleus ( Proton + Neutron ).
The electrons revolves round the nucleus in the well defined orbits and the size of nucleus is very small compared to the size of atom.
DRAWBACKS OF RUTHERFORD ‘S MODEL :.
According to Rutherford electrons revolve round the nucleus in well -defined orbits, but electrons being charged particles will lose their energy and finally will fall into the nucleus this make the atom highly unstable .
This was the major drawback which remain unexplained by him To over the drawbacks of Rutherford’s model Neil Bohr in 1912are purposed modified model of structure of atom.
He made the fallowing assumptions that only certain special orbits known as discrete orbits of electrons orbits the electrons’ d not radiate the energy. The energy is emitted or absorbed by atom only when an electron moves from one orbit to other
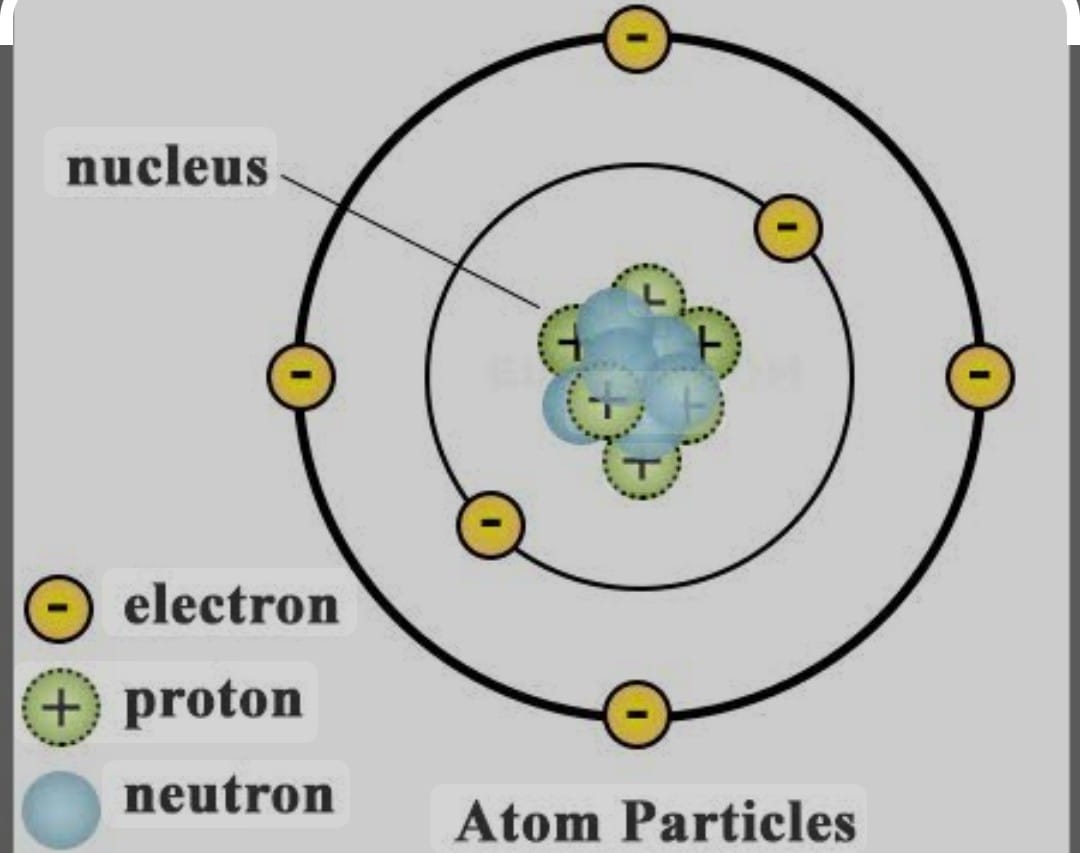
ATOMIC NUMBER :
It is the number of proton lying in the nucleus of any atom is called the atomic number. An atomic number is the identity of the atom, changing atomic number changing of the atom .
The atomic number is denoted by Z = number of proton for neutral atom no.of proton is equal to no of electron
MASS NUMBER :
It is the sum of total number of proton and neutron lying in the nucleus of atom .
Mass Number = Number of Proton + Number of Neutron Represented by A 13 Al ^27 here 27 is mass number and 13 is atomic number electron = proton = 13 number of neutron 27-13 = 14
17 Cl^35 => no.of proton = no.of electron = 17 >> no. of neutron = mass number- no of proton = 35 -17 = 18
11 Na ^23 no of proton= no of electron = 11 >> no. of neutron = 23 -11 = 12
DISTRIBUTION OF ELECTRONS
It is called Bohr Burry scheme of electron distribution. The filling of electrons in an atom is done in accordance to 2n²
where n is number of shell if n=1 (k) = 2 electrons ,
if n=2 (L ) = 8 electrons
if n=3 (M) = 18 electrons.
but last orbit has only not more than 8 electrons and
inner shell /antepenultimate shell has not more than 18 electrons.
for example
Na 11 = 2 (k) 8(L) 1(M) electrons distribution.
Al 13 k= 2 , L=8 M= 3 electros Ne 10 = k=2 M=2
VALENCE SHELL AND VALENCY :
From Bohr Burry sequence, we know that maximum number of election which can be accommodated in the outermost shell is 8 .
Every element tends to have 8 electron in the outermost shell ,in achieving 8 electrons atom can either gain electrons / loose electron .
The number of electrons lost / gained by the element in achieving 8 electrons determine the valency The electron in the outer most shell is called valence electrons
- C no. of electrons = 6 distribution of electrons 2,4 valence electrons =4 valency 4
- N no of electrons =7 distribution of electrons = 2 ,5 valence electron 5 valency = 3
- O no of electron =8 distribution of electrons= 2,6 valence electron =6 valency = 2
ISOTOPES :
They are the atoms of same elements having same atomic number but different mass number
isotopes of hydrogen is 1H¹, 1H², 1H³ isotopes of chlorine 17 Cl ^35 , 17Cl^37
Uranium isotopes is used as fuel in nuclear reactor , Isotopes of Cobalt is useful in treatment of cancer, an isotopes of iodine is used in the treatment of goiter. Carbon -14 is used in carbon dating .
RELATIVE ATOMIC MASS In any mixture of pure chlorine 75% of Cl^35 and 25% of Cl^37
average atomic mass = .75×35+ .25×37 = 35.5
ISOBARS :
Atoms of those elements which have the same mass number but different atomic number are called isobars.
Ca mass no.=40 and Ar mass no =40 but atomic number are different Na mass no =24 and Mg mass number =24.
Conclusion: Atomic Structure
Atomic Structure is the basic building block of the matter . Atom consist of nucleus at the Centre having proton and neutron with electrons orbiting around the nucleus with definite energy level. The arrangement of sub atomic particles determine the nature and behavior of the elements.
.Read More : Atoms and Molecules
Follow us on : Facebook