Acids Bases and Salts
Acids Bases and Salts is the important chapter of Chemistry /Science for class 10 Cover the complete syllabus of the class .The whole of the chapter covers the complete chapter in very easy language to understand for all students
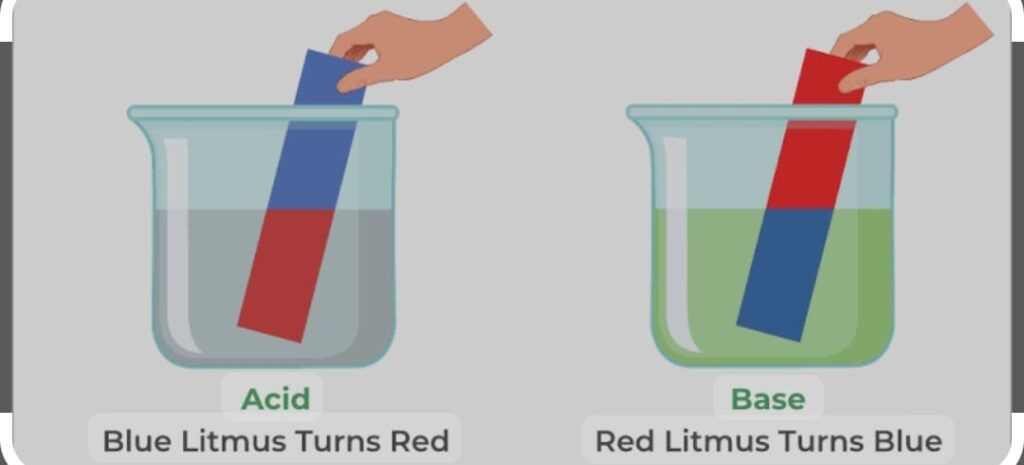
ACIDS are sour in taste and change the colour of blue litmus to red whereas bases are bitter and change the colour red litmus to blue.
Litmus solution is purple in colour extracted from lichen it is neither acidic nor basic ,natural indicators as red cabbage leaves it turns red /pink for acid green/yellow in case of base remain purple or blue in case of nuteral turmeric is also the natural indicator it remain yellow in acid /neutral and red in case of base if cloth is washed with soap or baking powder it changes in deep red colour .
There are some olfactory indicators if vanilla is added to basic solution there is no Odour but incase if added to acidic solution than vanilla odour continues, for onion if added to base smell is lost but in acid smell remains or does not change same is the case with clove.
REACTION OF ACID / BASES WITH METAL.

ACID +METAL ——–> SALT +HYDROGEN GAS
When sodium hydroxide is reacted with zinc it produces sodium zincate and hydrogen gas.
2NaOH + Zn ———–>Na2ZnO2 + H2
hydrogen gas obtained is collected by water displacement method.
H2 is determine when burning flint is taken near the gas it start burning with pop sound.
REACTION OF ACIDS ON METAL CARBONATES/HYDROGENCARBONATES:
When metal carbonate /hydrogen carbonate is reacted with dil.acid it produces salt carbon di oxide and water.
When sodium carbonate /sodium bi carbonate is reacted with dil HCl it gives the salt of sodium chloride, effervescence of carbon di oxide, water
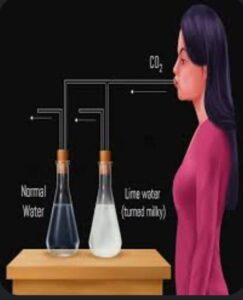
This CO2 when passed into lime water it changes to milky in colour of calcium carbonate if it continues than milky colour disappear forming calcium bi carbonate.
Na2CO3 + 2 dil HCl ———–> 2 NaCl (aq) + H2O (l) + CO2 (g)
NaHCO3 + 2 dil HCl ————> NaCl (aq) + H2O (l) + CO2 (g)
CO2 (g) + Ca (OH)2 ———–>CaCO3 (s) + H2O (l)
CaCO3 (s) + H2O + CO2 ————-> Ca (HCO3) 2 (aq)
NEUTRALISATION :When acid react with base it nullified the effect of each other and form salt and water .
NaOH (aq) +HCl (aq) ——-> NaCl (s) +H2O (l)
ACID +BASE ——–> SALT +WATER
REACTION OF METAL OXIDE WITH ACIDS :Take a small amount of copper oxide in a beaker add dilute hydrochloric acid in it slowly stir it we see the colour of solution becomes blue green and copper oxide is dissolved it is due formation of copper (II) chloride .
METAL OXIDE + ACID ——> SALT +WATER
CuO (s) +2 dil. HCl ————> CuCl2 +H2O
similar is the case with base and acid, such metal oxides are called basic oxides and when non- metallic oxide which is base react with CO2 to produce salt and water such nonmetallic oxide are acidic oxide in nature.
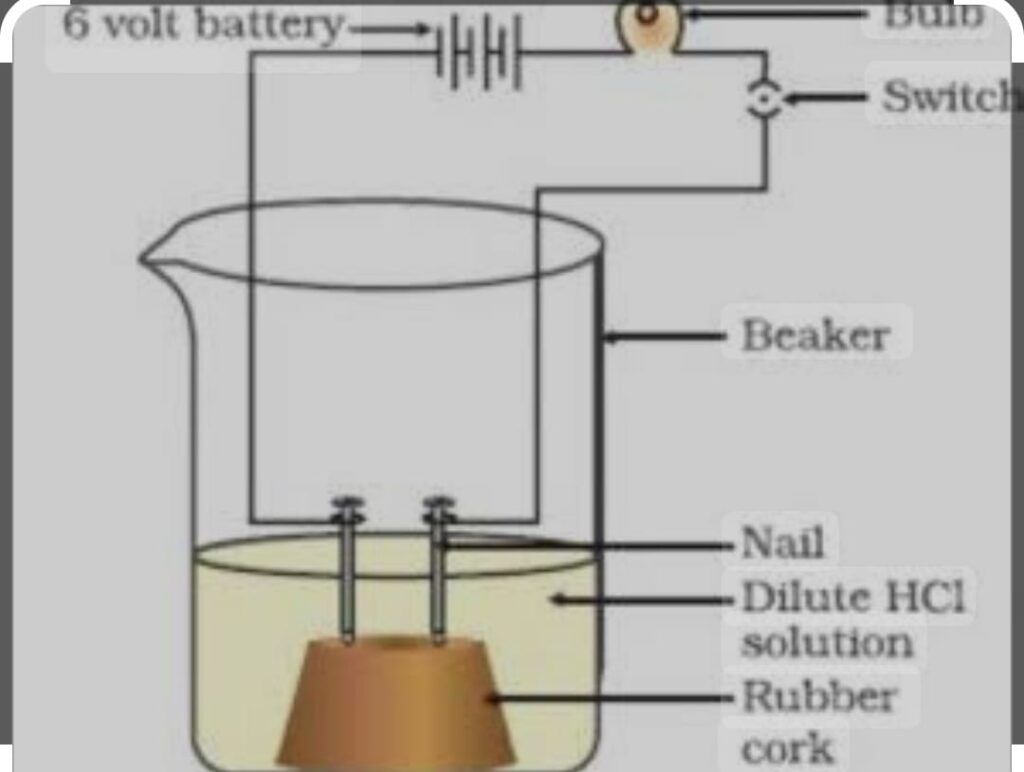
CONDUCTION OF ELECTRICITY : Take the solution containing dilute HCl / H2SO4, fix the two nails on a cork place the cork in 1 00 ml beaker connect the nails with the two terminals of 6 volt battery and pour the solution in the beaker as the switch is on bulb start glowing it shows that there is flow of electricity in solution through ions. As H+ ion present in acid produce hydrogen ion.
ACIDS / BASE SOLUTION IN WATER: Take small amount of NaCl add concentrate H2SO4 to it test the gas evolve with dry/wet blue litmus paper we see H+ ion in HCl is produced in presence of water it cannot be possible without water.
HCl + H2O ——–> H3O(^+) C l ^- as hydrogen ion cannot exists alone it combine with water molecule to form hydronium ion H3O^+.
NaOH (s) ——-H2O———> Na ^+ (aq) +OH ^- (aq)
KOH (s) ———–H2O ——–> K^+ (aq) + OH^- (aq)
Mg (OH)2———H2O ——–> Mg ^2+ (aq) + 2 OH ^-(aq)
bases always generate hydroxide ion in water ,the bases which are soluble in water are called alkalis they are soapy in touch ,bitter and corrosive.
ACID + BASES ——> SALT + H2O
HX + M-OH ———–> M-X + H-OH
H (aq) + OH (aq) ——-> H2O (l)
The process of dissolving an acid /bases in water is highly exothermic. We should be careful in mixing concentrated nitric /sulphuric acid with water.
Acid must always be added slowly to water with constant stiring.If water is added to concentrated acid the heat generated may cause the mixture to splash out may cause burn it may also break the breaker due to excessive heat.
Mixing an acid/base with water results in decrease in concentration of ions per unit volume such process is called dilution of acids or bases.
MEASUREMENT OF ACID /BASE STRENGHT The scale for measuring of hydrogen ions concentration in the solution is pH scale p stands for potenz in German means power. The scale is developed by Dr. Soren Sorensen the Danishscientist.
acids pH lies from 0 to 7 strength increases with decrease in number water) pH 7 Bases pH lies 7 to 14 strength increase with increase in number.
Substance ——————-pH
gastric juice 1.2
lemon juice 2.2
pure water 7
milk of magnesia 10
sodium hydroxide 14
IMORTANCE OF pH
i) As human body can survive within narrow range of pH 7 -7.8 ,rain water with pH of 5.6 is acid rain which is very harmful of aquatic life and plants .For plants healthy growth pH of soil should be specific.
ii) Our stomach produces HCl which helps in digestion of food but during indigestion the stomach produce much acid and causes pain and irritation to get rid of this milk of magnesia should be taken.
iii) Tooth decay starts when the pH is below 5.5 the tooth enamel pf calcium phosphate the hardest substance of the body it start corroded when pH of mouth gets below 5.5 bacteria in mouth produce acids by degradation of sugar and food particles that remain in mouth after eating food.
iv) some substances such Vinegar contain acetic acid ,orange/lemon have citric acid, tamarind have tartaric acid, tomato have oxalic acid ,lactic acid in curd, methanoic acid in ant /nettle sting .The common salt is sodium chloride but having impurities so called rock salt formed by sea water beds dried up which was done by Mahatma Gandhi in Dandi March.
SOME COMMON SALTS
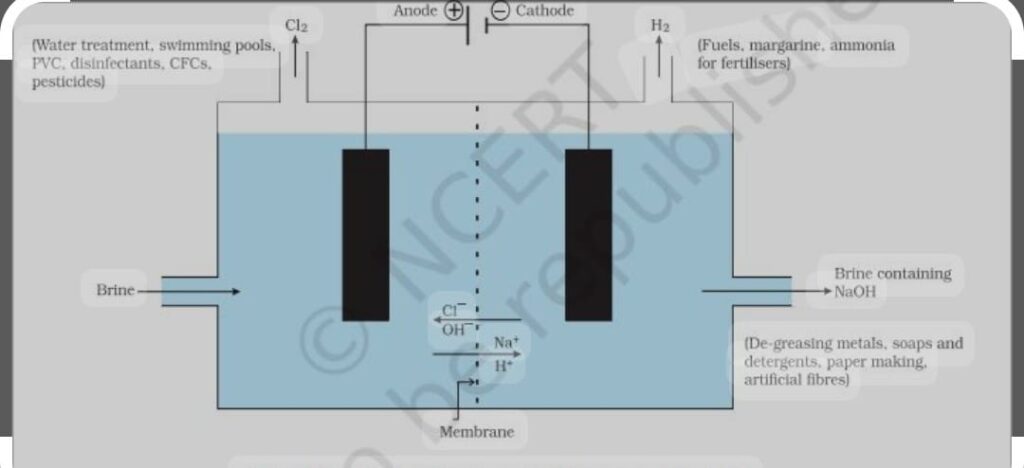
i) SODIUM HYDROXIDE (NaOH) When electricity is passed through the aqueous solution of sodium chloride/ brine which decomposes into sodium hydroxide it is chlor -alkali process.
Chlorine gas is given out at anode and hydrogen at cathode H2 gas is used for fuels, manufacture of NH3, Cl2 is used for water treatment, PVC disinfectant CFC, pesticides, NaOH for de greasing of metal, for manufacture of soap and detergents, paper making, artificial fibers.
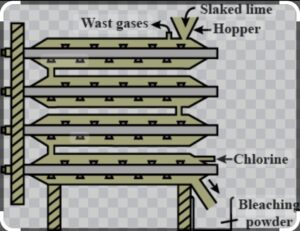
ii) The Chlorine gas is used in manufacture of bleaching powder when reacted with slaked lime Ca(OH)2 to form calcium oxy chloride/ bleaching powder CaOCl2.
Ca(OH)2 +Cl2 ———> CaOCl2 +H2O
It is used for cotton and linen in textile industry, bleaching of wood and pulp in paper and washed clothes ,oxidising agent in chemical industry, disinfecting of drinking water.
iii)BAKING SODA : It is mostly used in kitchen for making food crispy it’s chemical name is sodium hydrogen carbonate(NaHCO3)
NaCl +H2O +CO2 +NH3 ———->NH4Cl + NaHCO3
it is non corrosive, when heated
2NaHCO3 ——-heated ——–> Na2CO3 +H2O +CO2
It is used with mild edible oil tartaric acid when heated or mixed in water the CO2 produced during the reaction causes the bread / cake to rise making them softy and spongy. It is also antacids it neutralises the excess acid in stomach, used in soda acid in fire extinguishers.
Also Read: STRUCTURE OF ATOM Class IX

iv) WASHING SODA :Recrystallisation of sodium carbonate gives washing soda/basic salt.
Na2CO3 +10 H2O ——–> Na2CO3.10H2O
It is used in manufacturing of glass, soap, paper industry, borax, cleaning agent of domestic purposes, for removal of permanent hardness.
v) PLASTER OF PARIS: On heating gypsum at 373k it loses water molecules and becomes calcium sulphate hemi hydrate (CaSO4 1/2 H2O) called plaster of Paris used in supporting the fractured bones in the right posture if water is mixed it again changes to gypsum it is also used in making false ceiling and toys.
CaSO4.2H2O ———-373 k ——-> CaSO4 1/2 H2O
CaSO4.1/2 H2O + 3/2 H2O ———-> CaSO4.2H2O
WATER OF CRYSTALLISATION: It is the fix number of water molecules present in one formula unit of salt for example blue vitriol/hydrated copper sulphate CuSO4.5H2O, washing soda Na2CO3 .10 H2O , gypsum CaSO4.2H2O. green vitriol/ferrous sulphate heptahydrate FeSO4.7H2O.

CONCLUSION ACIDS BASES AND SALTS
Conclusion this chapter relates to basic idea of acids bases and salt which is suitable for the class 10 along with the preparation of common salts and its uses in our daily life .It also consist of importance of pH in different fields.
follow us on facebook
read more alkatopper
Follow us on Instagram