Carbon
Carbon that covers syllabus of class 10 Science related to chemistry gives the basic idea of Carbon, its nature and types .

Carbon
In the earth crust is about .02% and found in the form of minerals, in case of atmosphere it is .03% of carbon di oxide.
All living structures are carbon based it is present in paper, plastics, leather and rubber.
Carbon is the versatile element.
Carbon Covalent Bond : we know that atomic number of carbon is 6 it’s electronic configuration is 2,4 it is tetra- valent has 4 electrons in the outer most shell/valence shell now Carbon has to attain a noble gas configuration.
There are two possibilities one to gain four electrons which is difficult for nucleus to hold 4 extra electrons, carbon requires large energy.
If carbon loses 4 electron it also require lot of energy to remove 4 electrons. Carbon neither takes nor loses 4 electrons or valence electrons.
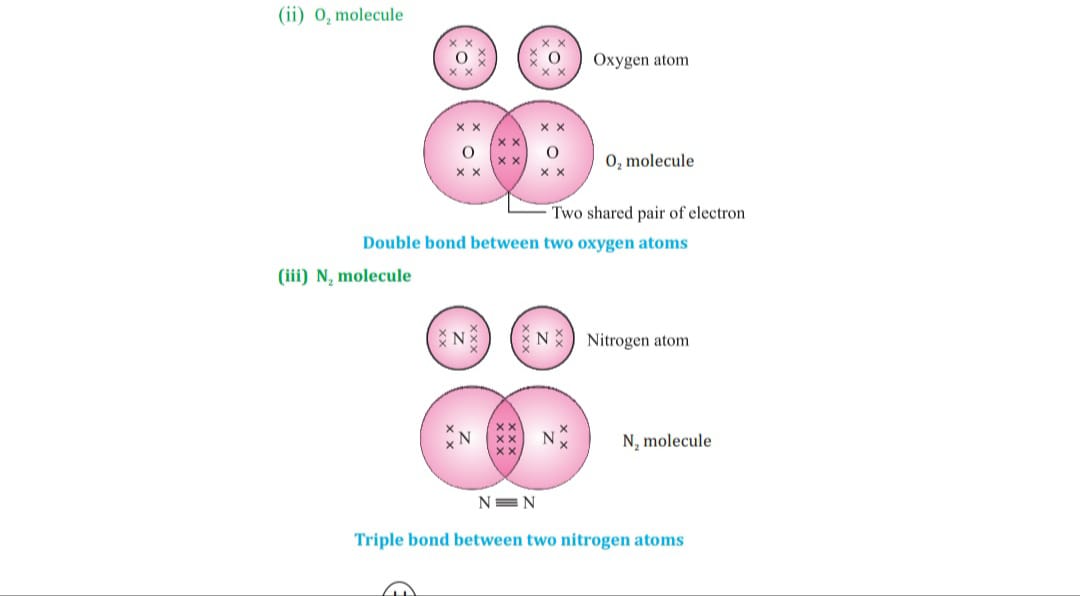
Carbon attains the noble gas configuration by sharing of electrons with the other atoms. This sharing of electrons of carbon is called covalent bonding.
The shared electrons belong to the outer shells of both atoms, which form noble gas configuration.
When two or more atoms share their valence pair of electrons to gain noble gas configuration ,
The number of shared pair of electrons may be one ,two ,three ,four electrons. Bond which is formed by sharing of electron pairs between two atoms is called covalent bond.
It has low melting point, boiling point because of weaker intermolecular forces these molecules are the poor conductor of electricity as no charge particles are formed. Some examples of covalent compounds. 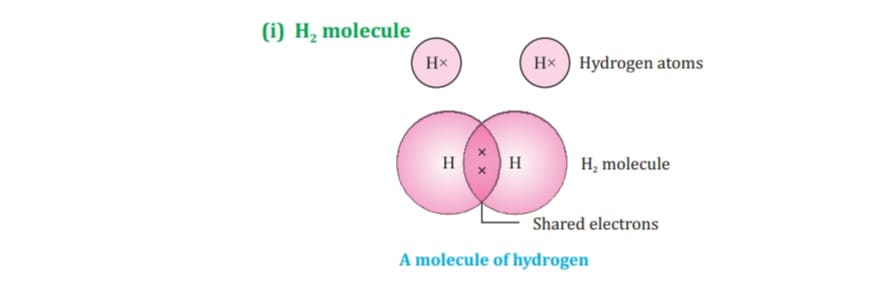
ALLOTROPES OF CARBON:
There are three allotropes of Carbon that is Diamond in this each carbon atom is bonded with four carbon atom in tetrahedral bond which makes it the hardest substance,
Diamond is electricity insulator but good conductor of heat and transparent in nature used making jewellery thermometer and glass cutting.
Graphite is another allotrope of Carbon in which each carbon atom is bonded to three other atoms of the carbon in hexagonal form bond the fourth valence electron can move so it is good conductor of electricity soft and opaque in nature .
Mostly used in pencils lead, electrodes and lubricant. Fullerenes having 60 carbon atom structure of soccer football
VERSATILE NATURE OF CARBON: There are three important properties of carbon atom which make it versatile enable carbon to form numberless compounds.
They are
a) Catenation
b) Tetravalency
c) Isomerism
Catenation is the property in which carbon atom forms bond with other carbon atoms making long, open, closed chains for example carbon, silicon has this property form numberless compounds with hydrogen.
Tetravalency the carbon atom has 4 electrons, it is capable of bonding with 4 atoms of oxygen, hydrogen, nitrogen ,sulphur ,chlorine and many other elements
The small size of carbon atom enable nucleus of carbon to hold the shared pair electron strongly and have stability.
SATURATED AND UNSATURATED COMPOUNDS OF CARBON.
The hydrocarbon are divided into open chain and cyclic /closed chain. 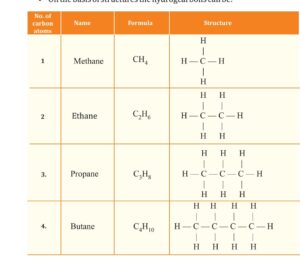
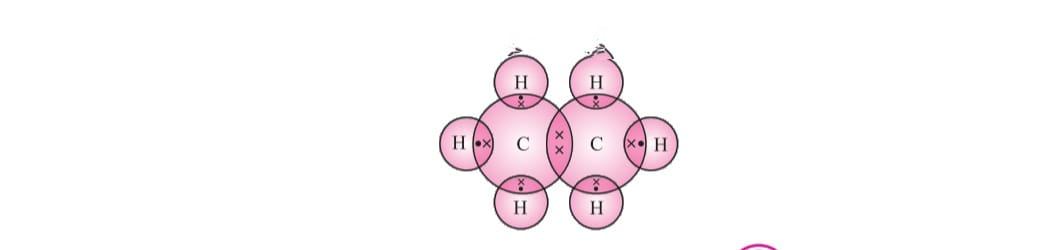
OPEN CHAIN:
The open chain of hydrocarbon classified into straight and branch chain.
The straight chain. further divide into saturated hydrocarbon as
ALKANE general formula CnH2n+2
as Methane CH4, Ethane C2H6.
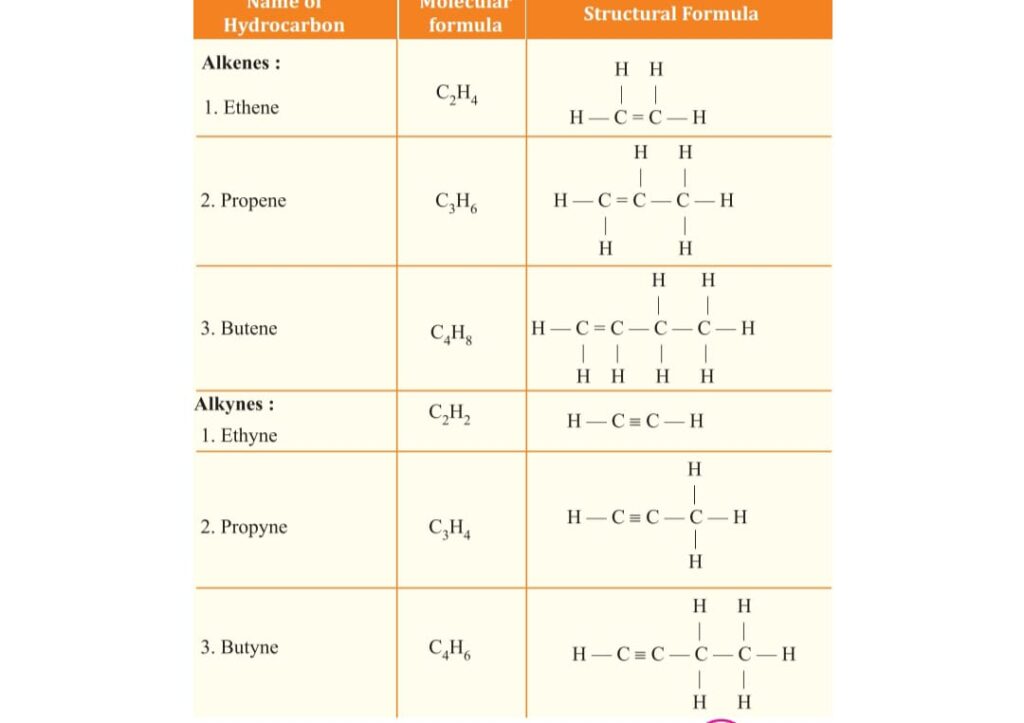
unsaturated are as ALKENE general formula CnH2n
as Ethene C2H4, Propene C3H6,
ALKYNE general formula CnH2n-2
such as Ethyne C2H2 , Propyne C3H4 .
ALICYCLI C CHAIN:
It has closed ring of carbon chain as Cyclo-Propane asC3H6.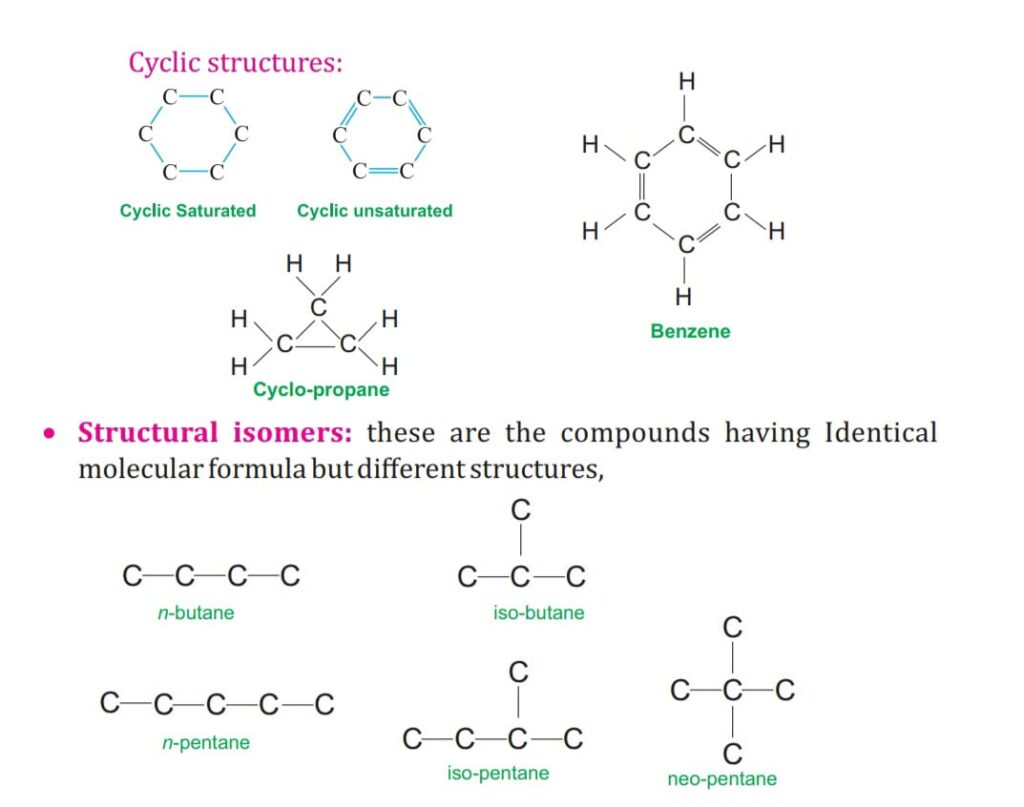
It is to be noted that for alkene or alkyne there are double or triple bond for which two carbon are required therefore the first member start from the group as ethene and ethyne.
HOMOLOGOUS SERIES:
It is a series of compounds in which the same functional group substitutes by hydrogen in the carbon chain such as CH3OH, C2H5OH etc.
The successive members differs – CH mass differ by 14.
The chemical properties depend on the functional group so they have similar chemical properties but different physical properties.
The physical properties vary among the members of series due to difference in their molecular masses. Melting and boiling point increases with the increase in molecular masses.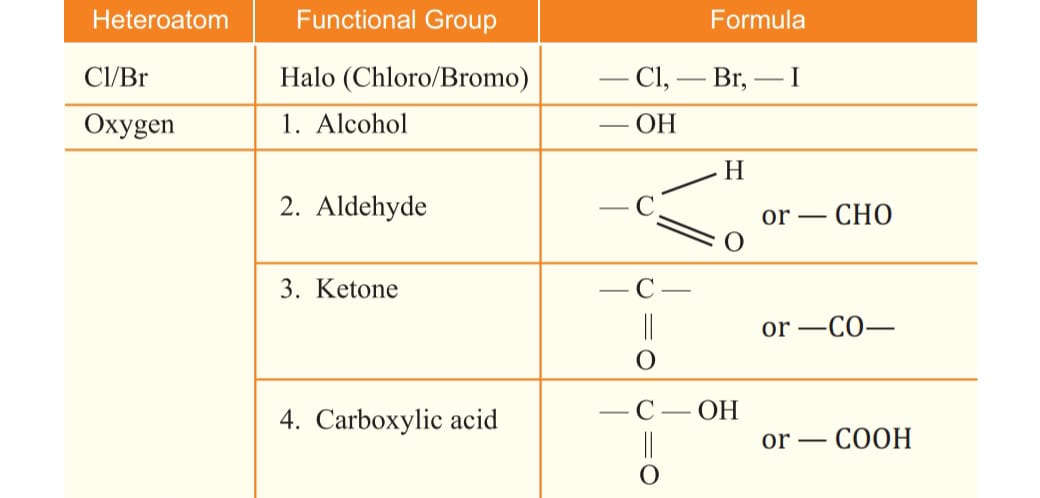
CHEMICAL PROPETIES OF CARBON COMPOUNDS:
a) COMBUSTION of Carbon generally burns or oxidize in air that gives carbon di oxide ,water ,heat and light.
CH4+ O2 ——————–>CO2 +H2O +heat+ light
Saturated hydrocarbon burns with blue flame but yellow flame in limited supply of air.
Unsaturated hydrocarbon burns with sooty flame but when coal is burnt emits Sulphur or nitrogen which is responsible for acid rain.
OXIDATION Compounds of Carbon : When alcohol are oxidized presence of acidified potassium dichromate or alkaline potassium per magnate it is converted into carboxylic acids.
C2H5OH + (O)——–acidifiedK2Cr2O7/ alkaline KMnO4—->CH3COOH +H2O
the step of oxidation as follows when alkane is oxidized changes to alcohol on further oxidation it changes to aldehyde on further oxidation it changes to carboxylic acids
CH4 —- oxidized —>CH3OH —-oxidized–>HCHO—oxidized_>HCOOH
all oxidation takes place in presence of acidified K2Cr2O7/ alkaline KMnO4.
ADDITION REACTION of Carbon Compounds
In this addition of Hydrogen takes place with unsaturated hydrocarbon in the presence of Nickel/platinum/palladium as catalyst at temperature of 473 deg C
In this triple bond changes to double bond and then to single bond compounds such as alkyne changes to alkene and then to alkane this is called hydrogenation.
vegetable oil converted to vegetable ghee by this process. Hydrogenations slow the rancidity of the vegetable oil. It is advisable to use oils with unsaturated faffy acids for cooking
CH2= CH2 + H2 ——— Ni/473 deg C —–> CH3-CH3
SUBSTITUTION REACTIONS of Carbon Compounds: This reaction takes place in presence of sunlight where hydrogen attached to carbon can be replaced by another atom or group of atoms.
If chlorine react with alkane in presence of sunlight it forms halo alkane as process continue till last hydrogen is replaced this process is called halogenation in case of chlorine it is chlorination.
CH4+Cl2 — light/520 deg C——–>CH3Cl +HCl Chloromethane)
CH3Cl +Cl2 ————-..> CH2Cl2 +HCl
CH2Cl2 +Cl2 ———————-.> CHCl3 + HCl Trichloromethane)/ chloroform
CHCl3 +Cl2 ————————–.> CCl4 +HCl (Carbon tetrachloride)
ETHANOL: The melting point of ethanol is 156k, boiling point is 351k, it is soluble in water, taste of burning, the consumption of dilute ethanol causes serious health problems in case of taking pure ethanol is very dangerous.
C2H5OH +Na———> C2H5ONa +H2 (sodium ethoxide)
C2H5OH ——- dehydration/ conc H2SO4——> C2H4 + H2O (ethene) 443k
Ethanol is used as antifreeze in radiators of automobiles, in the preparation of transparent soap ,cosmetics ,laboratory reagent, alcoholic beverages, medicines and tonics.
ETHANOIC ACID/ACETIC ACID (CH3COOH) ;it’s boiling point is391k ,sour in taste miscible in water and freezes at 29k .In pure form it is called glacial acetic acid ,it is called vinegar when solution contain 3 to 4 % of acetic acid.
CH3COOH+ Na2CO3 ———-> CH3COONa+CO2 +H2O (sodiun ethanoate)
CH3COOH +Na ———–>CH3COONa +H2
CH3COOH +NaOH ———–.> CH3COONa+ H2O
CH3COOH +NaHCO3 ——-.> CH3COONa+CO2 +H2O
CH3COOH +CH3CH2OH ———-> CH3COOC2H5 +H2O (ethylacetate/ester )
When carboxylic acid reacts with+ alcohol it form ester this process is called esterification (above last equation is the example)
general equation:
RCOOH + ROH ——– conc H2SO4 —–.>RCOOR +H2O
HYDROLSIS of Carbon Compounds: Heating the ester compound in presence of acid or base it gives back alcohol and salt of carboxylic acid it is also called saponification when it is made alkaline hydrolysis.
CH3COOC2H5 + NaOH——–>CH3COONa +C2H5OH
CH3COOC2H5 ——dil H2SO4——–.> CH3COOH +C2H5OH (not saponification)
SOAPS AND DETERGENTS AND CLEANING ACTION ;
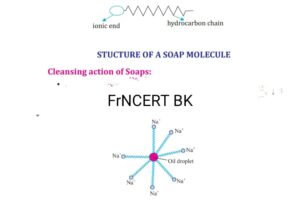
Soap is the sodium/potassium salt of long chain fatty acids such as Palmitic acids ,steric acid C15H31COOH OR C17H35COOH soaps are effective with soft water only but remain ineffective with hard water .
Detergents are ammonium /sulphonate salts of long chain of carboxylic acids they form leather with both soft as well was hard water the ionic part is hydrophilic /water attacking whereas hydrocarbon part is hydrophobic/ water repelling it is the part which is present in soap.
Most of the dirt is oily and hydrophobic attack to the dirt, ionic part is surrounded with water molecules it forms the radial structure called micelles. The emulsion is formed by soap molecules than cloth are agitated mechanically to remove the dirt from cloth hence cloths are cleaned.
SCUM: we know that in hard water there are impurities of magnesium or calcium salts that react with soap molecules form insoluble product called scum which do not allow cloth to clean for this we use detergents which do not form scum with hard water cloths are cleaned. Soaps are biodegradable and environmentally friendly while detergents are not .
NOTE all figures and tables taken from NCERT book
Conclusion: Carbon
carbon and compound is the chapter which gives the basic idea of carbon used in daily life along with fundamental knowledge about the diversity , structure , properties and significance of organic chemistry.
Read More : Chemical Reaction and Equation
Follow us on : Facebook