IS MATTER AROUND US PURE
Is Matter Around us Pure is the 2nd Chapter of chemistry of class 9th covering the whole syllabus of the chapter which gives basic idea of chemistry introduction.

Pure means substances without impurity but in science all things are mixture of so many substances so they are not pure . Milk consist of water and fats .
In science pure substances are those in which all the elements have same chemical properties and made up of same kind of elements .
SUBSTANCE is a kind of matter that cannot be separated into other kind of matter by any physical process a pure substance are made of same element .
MIXTURE ;
It is the substance in which two or more substance (element / compound ) are simply mixed together in any proportion eg Air is a mixture of many gases
There are two types of mixture
a)Homogenous Mixture :
It has no visible boundaries of separation between the various constituents eg sugar in water it has a uniform composition through out the mass .
b)Heterogenous Mixture :
These types of mixtures have visible boundaries of separation the various constituents eg mixture of sugar and sand it does not have a unform composition throughout mass
SOLUTION :
It is homogeneous mixture of solute and solvent. The component which is in small part is solute and the component in large amount is solvent .
Sugar + Water = solution
here sugar is solute and water is solvent .
Solutions are classified in three categories :
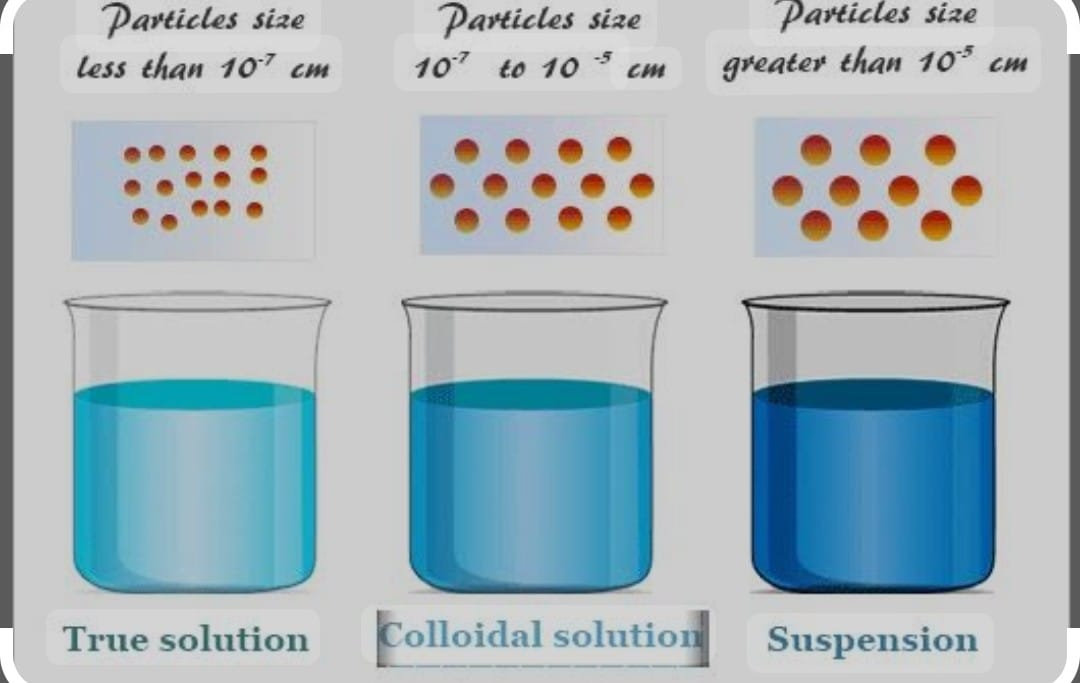
a) True solution in which the size of the particles is less than 10 -9m , particles of solute cannot be seen through naked eyes ,
They are Homogenous mixture which cannot be separated by filtration ,
They are transparent in nature, very much stable do not settle down, they do not show Tyndall effect
,This type of solution diffuse rapidly through filter paper or parchment paper .
b) Colloidal Solution :
In this kind of solution the size of solute particles lies between 10-9 to 10-6 m , the particles of solute cannot be seen through the naked eyes ,
Itis heterogenous mixture ,cannot be separated by filtration, Translucent in nature , stable in nature , shows Tyndall effects ,
It passes through filter paper and cannot pass through parchment paper
Tyndall Effect; It is the scattering of light in colloid. This effect is visible when light passes through a colloid such as smoke or fog the light been is visible. In this process the light collides with the particles of colloidal and scatters in all directions
c) Suspension solution :
Size of the solute particles is more than 10-6 m , particles can be seen through naked eyes
,Heterogenous mixture ,can be easily separated by filter paper ,Opaque in nature , unstable in nature , may or may not show tyndall effects ,
They neither passes through filter /parchment paper .
SOME COMMON COLLOIDS :
Liquid / solid+ Gas = Aerosol (Fog, Cloud , smoke )
Gas + Liquid = Foam ( Shaving Cream ) ,
Liquid + Liquid = Emulsion ( Milk , Face Cream )
Solid+ Liquid =Sol (Mud , digene ) ,
Gas + Solid= Foam (rubber, sponge , Foam )
Liq³uid + Solid =Gel (jelly , Cheese )
Note : gas in gas is not colloids
CONCENTRATION OF SOLUTION :
Mass by % mass =mass of solute x 100/ mass of solution
Mass by % volume = Mass of solute x100 /volume of solution
METHODS OF SEPARATION OF MIXTURE :
Evaporation :
The basic principle out of the two components of mixture one can evaporate which has low boiling point than the other having high melting point. In the mixture of dye ( higher bp) and water .Out of water and dye water evaporates dye is left behind .
Centrifugation :
The basic principle of the separation of substances or particles on the basis of their density, when mixture is rotated very fast the denser particles are forced at the bottom and lighter particles remain on the surface eg used in diagnostic lab for blood test and urine test , used in home /dairies to separate butter from cream , Used in washing machine dryers to squeeze out water from clothes
By Separating funnel :
when two immiscible liquids ( do not dissolve with each other) can be easily separated by this funnel . Let us suppose water and oil are mixed and put in the separating funnel if we open the stop cock first water will be removed after than oil can be collected .In the same way extraction of iron ore takes place lighter slag is removed from molten iron .
SUBLIMATION :
Out of the two component one will sublimate and other is left behind In the mixture of NH4Cl and NaCl when it is heated NH4Cl being sublimate evaporates and NaCl is left some other sublimate are Camphor /naphthalene /anthracene/NH4Cl.
CHROMATOGRAPHY :
The coloured component of a mixture can be separated by using adsorbent on which they are absorbed at different rates .( Surface absorption is adsorption ) when water/any suitable solvent moves up the chromatography paper ink with two different colour separates because both colours are absorbed at different rates eg it is used to separates colours of dyes /to separates pigments from natural colours like chlorophyll/separate drugs from blood.
DISTILLATION : It is used to separate the miscible mixture liquids having different bp followed by condensation out of two component one has lower bp and other have high bp ,this process is used for separating two or more miscible liquids ( see fig bk )
When mixture of acetone and water is heated acetone having less bp boils and moves to delivery tube within which it condenses back to liquid with the help of condenser clamped to it in this way acetone is separated water is left behind distillation flask . We must use fractional distillation if there are more than two miscible liquids. Petroleum is separated into paraffin wax , lubricating oil , diesel , Kerosene ,petrol and petrol gas is separated by this method.
FRACTIONAL DISTILLATION OF AIR :
Air is separated by this method in which air is compressed and cooled with becomes liquid air than further allowed to warm up slowly in the fractional column and air is separated at different heights .
Important application
a) In oil refineries to separate the crude oil into useful substances
b) In is used for the separation of oxygen , liquid nitrogen and argon from air.
CRYSTALLISATION :
It is the method to remove the impurities from the mixture by first dissolving in a suitable solvent and then crystallizing out the one component
Copper Sulphate crystals which are impure are first dissolved in H2SO4 than heated to saturated solution. This solution is left overnight so only CuSO4 crystals on filter paper.
Crystallization is better than evaporation because some solids decomposes or charred upon heating to dryness during evaporation ( Sugar ),some impurities remain dissolved in solution after filtration.
On the evaporation these impurities do not evaporate and remain with the mixture. It is used for the purification of salt from water , separation of crystal from impure crystals ( alum ,copper sulphate )
WATER PURIFICATION IN WATER TREATMENT OF PLANT :
Reservoir which contain impure water which transfer to sedimentation tank in which solid and heavy particles settle down than further goes to loading tank with the alum etc sedimentation of suspended impurities than it goes to filtration tank where all impurities are filtered after that it goes to chlorination tank in which chlorine is added to kill germs after water is supplied to homes.
PHYSICAL CHANGES :
It is the change that are easily reversible ,no new products are formed and only change in state take place eg melting of ice.
CHEMICAL CHANGE:
Itis the change that is not easily reversible where new product is formed ,often heat , light , sound or fizzing occurs ,and electricity may be produced such as wood burning .
ELEMENTS are made of same type of atoms which are classified in metals, non metals and metalloids.
METALS are the lustrous, malleable , ductile, sonorous , good conductor of heat and electricity eg gold iron
NON METAL are the non lustrous, non malleable ,non ductile ,non sonorous , bad conductor of heat and electricity eg oxygen , phosphorous
METALLOIDS have intermediate properties between metals and non metals eg boron , germanium ,silicon .
MIXTURE : Elements /Compounds are simply mixed so no new substance is formed , they do not combine in fix ratio, it shows the properties of its components , which can easily separated by mechanical method which is suitable eg sugar in water , oil in water
COMPOUND are the substances which are reacted together with each other to make new substance ,the composition of of the component is fixed they combine together in a fixed ratio according to their masses , it does not show the properties of component elements they can not be separated from each other by simple mechanical methods such as water .
Conclusion: Is Matter Around Us Pure
Is Matter around us pure is the chapter which basic idea of terms of chemistry like solution it’s type , mixture and it’s types , definition of element, compounds, metals and non metals along with idea different methods of separation. of mixtures .
Follow us on: Facebook
Read More: Matter in our surrounding
Read More : Atoms and Molecules