Matter in our surroundings
Matter in our surrounding is the 1st of chapter of Chemistry that gives basic idea of matter that covers the syllabus of class 9th
MATTER ;
It is anything which has mass and occupies space and it offer resistance if any force is applied to it .
Physical Nature :
Matter is made of particles and they are very small .
Characteristics
:a) Particles of matter are continuously moving because they posses the kinetic energy as the temperature rises the particles move faster because KE increases .
b) Particles of matter have space between them when we add salt to water the particles of salt goes into the space between the particles of water this show there is space between the particles of matter .
c) Particles of the matter attract each other when we open a water tap and try to break the stream of water by our finger but stream remain together it means that particles of matter attract each other with certain force ,
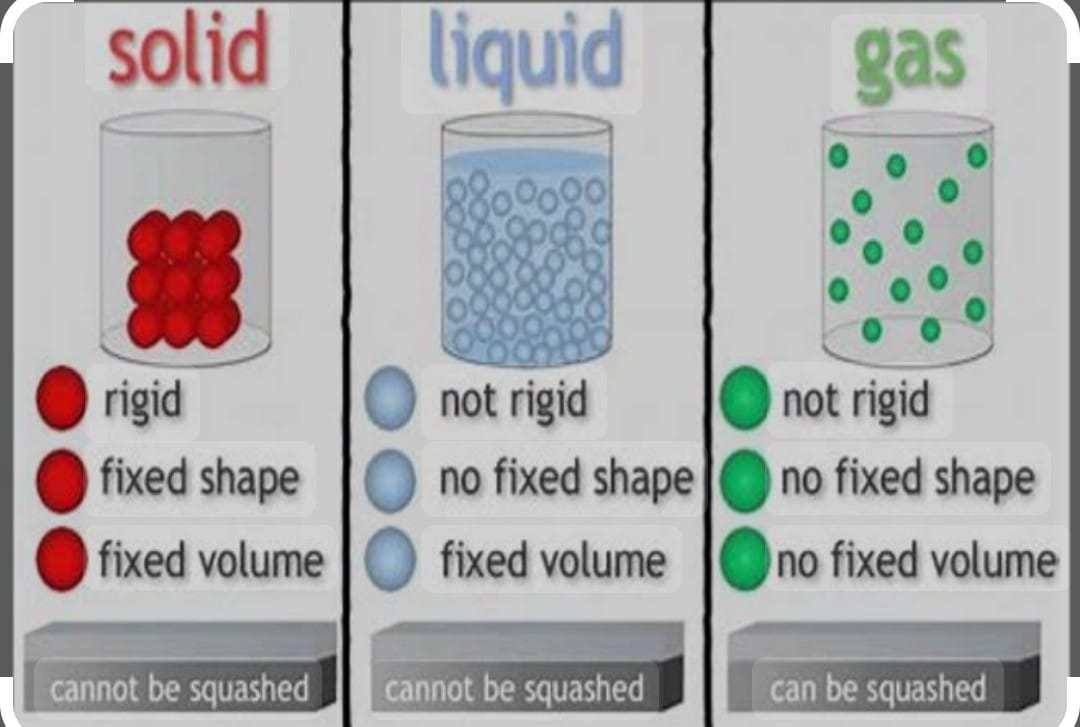
Incase of solid the space between the particles and Kinetic Energy is minimum. incase of liquid it is intermediate while in gases they are maximum.
Force of attraction is maximum in solids , intermediate in liquids and minimum in gases
STATES OF MATTER;: There are three states of matter i) Solid ii) Liquids iii) gases
SOLID :
They have definite shape, distinct boundaries ,Rigidity and incompressibility with definite volume .
Exception :
In case of rubber band being solid it changes it’s shape when force is applied and regain its shape as the force is released. As the kinetic energy of the particles of solid is very less than also it has fixed and rigid shape .
We can compress sponge as it has pores and air is in them but they are solid ,Sugar is in small crystal form being solid takes the of container
LIQUID STATE :
It has tendency of fluidity as they are not rigid in nature with low compressibility it has no definite shape and boundaries they take the shape of container in which it is placed with definite volume .
The force of attraction between the particles of liquid is less so it keeps the volume of liquid as it has definite volume but no fix shape it takes the shape of container
Some gases like O2 and CO2 from the atmosphere dissolve in water therefore aquatic plants and animals are able to breathe and able to survive .
Diffusion is the inter mixing of two or more substance it is more incase of liquid than solid due to free movement of the particles
GASES STATE :
They are highly fluidity ,with high compressibility no definite boundary, no definite shape and volume
The particles of gas are free to move in any direction so gases can flow ,it don ‘t have fix volume and occupy all the space available to it . Pressure of gas is the force applied on the walls of container by the irregular moving particles of gas .
CHANGE OF STATE :
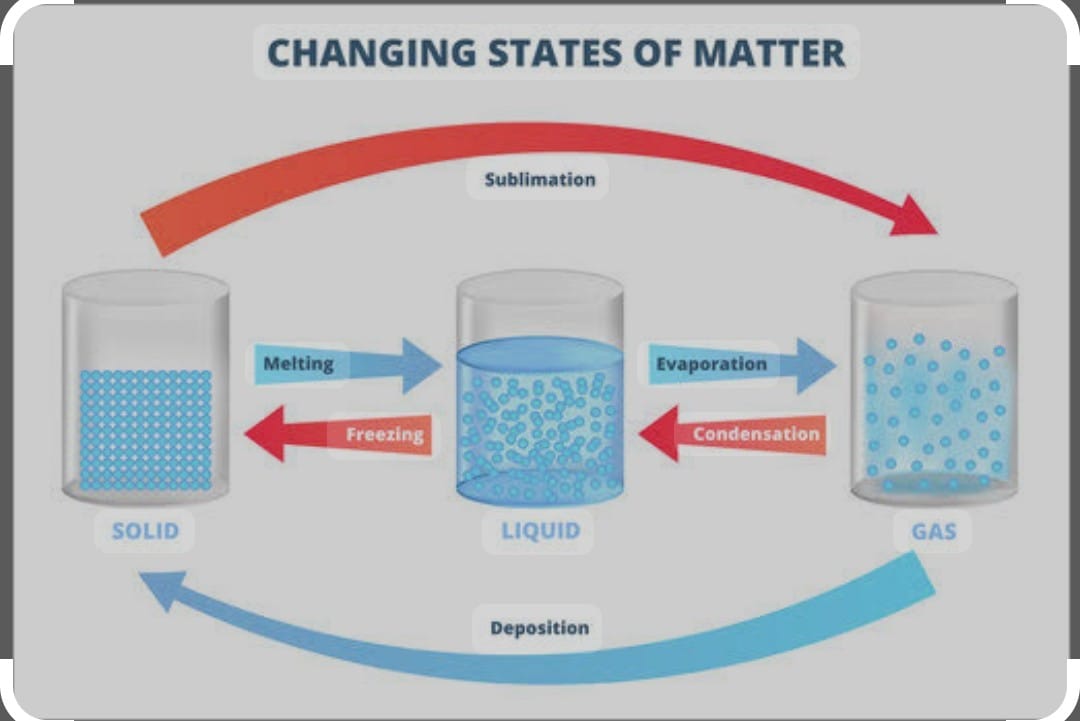
Water can exist in three state ie solid -(ice) liquid (water) gas (water vapour)
ice –heated —-. > water —— heated ——-> liquid
Physical state of matter can be changed my changing the temperature
MELTING POINT :
The temperature at which a solid melts in liquid state at atmospheric pressure is called melting point .
Melting point of ice is 0 deg c/ 273k .It is to be noted that during melting the temperature of ice does not change though heat is supplied continuously because of latent heat of fusion which is used to overcome the force of attraction between the particles. At 0 deg c water particles have more energy than ice .
LATENT HEAT OF FUSION :
It is the amount heat required to change the state of 1 kg solid into liquid state at its melting point without change of temperature.
BOILING POINT :
The temperature at which a liquid boils to form vapours at the atmospheric pressure is called boiling point for water 373k /100 deg c
LATENT HEAT OF VAPOURISATION;
The amount of required to change the 1 kg of water into vapours state at it’s boiling point , atmospheric pressure without change in temperature because heat is supplied to change the state from liquid to vapour state by overcoming the force of attraction between the particles
The energy of water vapour at100 deg c is much more than water at 100 deg c therefore steam is more dangerous than water .
SOLD STATE —- heat ——> LIQUID STATE ——- heat ——> GASEOUS STATE when gas is cooled it changes into liquid is called condensation further cooled becomes solid called freezing
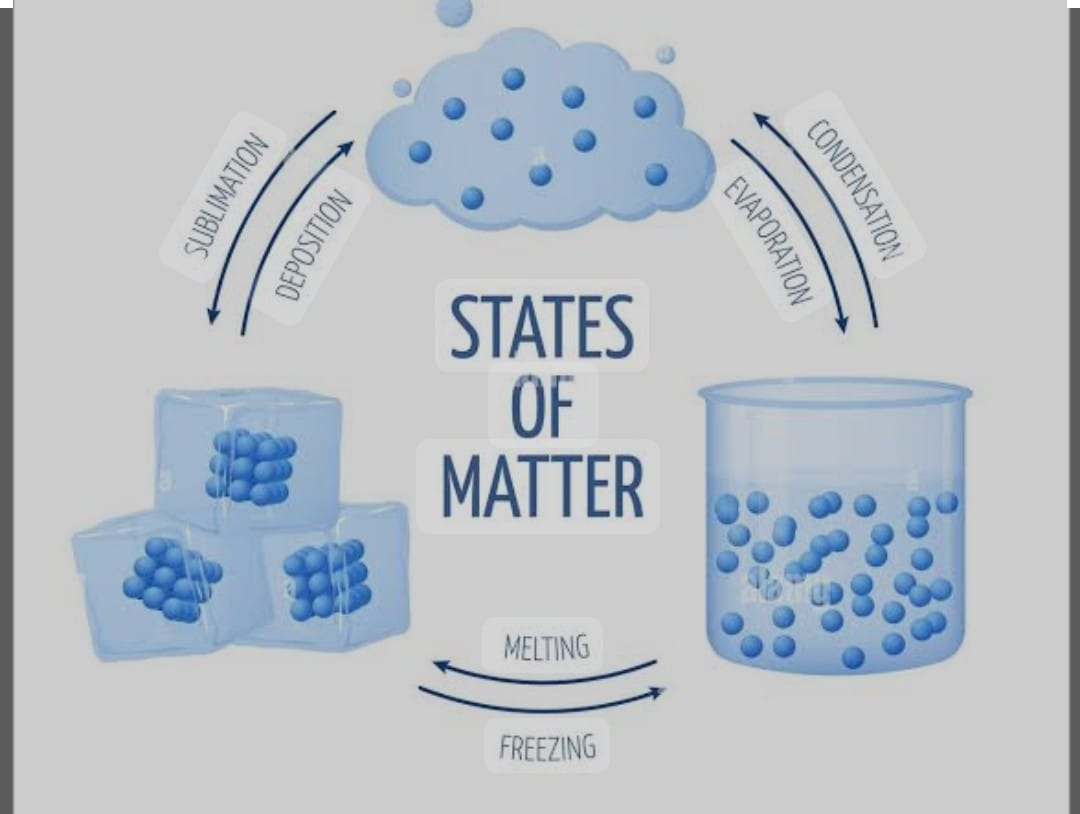
SUBLIMATION :
The change of solid directly into vapour on heating and of vapour into solid on Cooling without passing through the intervening liquid state is called sublimation .
When camphor /ammonium chloride is heated in china dish covered by a inverted funnel the vapours of ammonium chloride are converted into solid NH4Cl on coming in contact with the cold inner walls of funnel . Naphthalene balls sublimate with duration of time.
EFFECT OF CHANGE OF PRESSURE :
If we compress a gas in a cylinder , the distance between the particles of gas is reduce and finally gas is liquefied on the lowering temperature . On increasing the pressure the particles of gas comes very close .In case of solid CO2 /dry ice is changed into CO2 gas without changing into liquid when pressure is reduced at atmosphere .
SOLID ———-> LIQUID (melting ) ————> SOLID (freezing)
SOLID ————sublimation———> Gas————– sublimation ——-> SOLid
LIQUID ——— evaporation ——-> GAS ——– Condensation ———> LIQUID
EVAPORATION :
It is surface phenomenon in which liquid changes into vapors at any temperature below the boiling point, have higher kinetic energy than others particle Particles so they break the forces of attraction between the particles and escape from the surface of liquid in the form of vapors
FACTORS AFFECTING THE RATE OF EVAPORATION
a) Exposed surface aera on increasing surface aera of liquid the rate of evaporation increases
b) Increase in Temperature the kinetic energy of particles of liquid increases hence evaporation increases
c) If Humidity in air is low then evaporation is high if it is than it is less . d) Wind speed is more evaporation is high.
Evaporation always causes cooling because when evaporation takes place it takes latent heat of vaporization from the surroundings which on losing heat get cooled . the function of Desert cooler
When we put acetone / petrol on our hands it gets vaporized by taking heat from our hand so we feel cool
We should wear cotton clothes in summer to keep us cool and comfortable as cotton is good absorber of water so it absorb sweat from the body and expose to air by the evaporation of sweat we feel cool.
often people sprinkle water on the ground during summer this water takes heat from the ground and surroundings air evaporate making the place cool
Conclusion : Matter in our Surrounding
Matter in our Surrounding is the first Chapter of Chemistry of Class 9th it gives basic idea about Matter, States of Matter , Melting point, boiling point , change in states ,along with sublimation, and evaporation ,it’s factors and some practical example which prove worth to students and readers.
Follow us on: Facebook
Follow us on : Instagram
Read More : Is Matter Around Us Pure
Read More : Atoms and Molecules