Metals Non Metals and Carbon Compounds Questions
Metals Non Metals and Carbon Compounds Questions is the unit of class 10th CBSE from Science consist of Year wise 2018 to 2024 topic and Chapter wise questions

Metals Non Metals and Carbon Compounds Questions
Related to
Follow us : Facebook
Read More : Life Process Important Questions
METALS AND NON METALS

- Why metals become dull when exposed in air? Name two metals which are ductile and malleable .
- What is thermal conductivity of metal? Name the metal poorest conductor of metals.?
- a)Arrange the following in decreasing order of electric conductivity Mercury , Copper, Tungsten ,Aluminum . b) arrange decreasing order of reactivity Cu ,Al , Ca , Au.
- Metals are sonorous explain. Name the non- metals which are solid at room temperature. Name the metal which is found in abundant in the earth crust. Name the metals having low melting point. What is the general nature of metal oxide?
- A non-metal X exists in two different form Yand Z ,Y is the hardest natural substance where as Y is good conductor of electricity find X,Y,Z.?
- Metals Non Metals and Carbon Compounds Questions :Name one metal and one non- metal in liquid form . Name Two amphoteric oxide.(2024)
- What happens when Mg is burnt in ignition temperature.? *What happens i) Fe and ii) Cu is heated separated at very high temperature ? Name the metals which do not react with air at high temperature.
- What happens when metal react with water write the reaction ? Name the metals that react with hot water and steam give the equation in each case
- Name the metals that react violently with water? Name the metals that does not react with dilute acids . Name the compound which is formed when reacted with hydrogen.
- What happens when a piece of Ca react with water why does it float over the surface of water write the reaction . write the reaction of iron with water ?
- Why Na ,K, Ca metals form hydrides by combing with H2 gas but other metal does not
- A element M on heating with oxygen form M2O this dissolve in water and turn red litmus blue substance Name M
- A metal X on heating in air does not burn but acquires black coating substance Y name X and Y with the reaction.
- what happens when Zn is reacted with CuSO4. write the reaction..
- P ,Q,R,S,T, represent metals in decreasing order of their reactivity series which of the metal is likely to found in free state and which will be in the top of the series.(Important logical Question Metals Non Metals and Carbon Compounds Questions)
- Metal A has two oxide first AO second is A2O ,first one is neutral and other is acidic in nature is ” A” metal or non-metal.( Logical Question Metals Non Metals and Carbon Compounds Questions )
- Name the metal that react with dilute HNO3 give the reaction , Why does Cu does not give H2 gas when react with dilute H2SO4
- What is electrovalent compounds ,show the formation of NaCl by Na and Cl. ? what can you say about the solubility of electrovalent compounds,
- Write electronic configuration of element having atomic number 11 , 13
- Why ionic compound are hard crystalline solids, Name the metals never found in free state.
- a)Which metal is found as oxide, sulphide in earth’s crust . how are they extracted Name the chemical process used for obtaining metal from metal oxide.. b) why are carbonate and sulphide ores are usually converted into oxide during extraction.
- write the equation when manganese di oxide is heated with aluminium powder.
Metals Non Metals and Carbon
Compounds Questions
- What is corrosion ? Name the metals which do not corrode . Is corrosion advantageous if so than how ?
- Name the metal corrode in atmosphere , Why does silver article become black when left in air name the compound formed and why copper articles becomes greenish if left in air what compound is formed.
- Give one word for statement a)Metal oxide behaves as basic and acidic (2024) b) Iodine a non- metal is shining 2024 Oxides of Aluminum and Zinc is—— if reacted with HCl or KOH gives ——–.(Metals Non Metals and Carbon Compounds Questions year 2024)
- Write the chemical reaction a)when steam is passed over aluminum/ red hot iron b) CO2 is compressed in water at high pressure c) A copper plate is dipped in the solution of silver nitrate it becomes black why? d) Classify the acidic and basic oxide Na2O, SO2 ,MgO ,CO2 e) ZnS is heated in air (Important Reactions Metals Non Metals and Carbon Compounds Questions )
- a)Why no hydrogen gas is evolved when metal react with dilute nitric acid . give the name & reaction of the metal that reacts with it b) Why do ionic compound do not conduct electricity in solid state..
- Aluminium is used in making utensil Which of the following property i) Good thermal conductivity ii) good electrical thermal conductivity c) Ductility iv)high melting point .
- Which of the following is not ionic compound KCl/ HCl/CCl4/ NaCl.
- Which of the following property of ionic compound i) Solubility in water ii) Electric conductivity in solid state iii) high melting and boiling point iv) elctric conductivity in molten state
- Galvanization is the method of protection of iron from rusting by coating with thin layer of which metal.
- Stainless steel is a mixture of a) Ni and Cr b)Ni and Cu
- Na/ Ca/Fe/Cu which of these elements obtained by electrolysis
- 2 ml of conc. HCl and conc. HNO3 are mixed in ratio 3:1 taken in three test tubes A, B,C a small metal piece is added in each test tubes metal in A ,B remain as such but metal in C completely dissolved which metal may be in A and B Al/Cu/Au.
- Alloy is element of homogenous mixture/ heterogenous mixture
- During the electrolysis of Znic it deposit at cathode/ anode/ remain in solution.
- Na/P/Ca is very soft cut with knife react vigorously with water and kept in kerosene oil
- Which of alloy contain mercury stainless steel/ Znic amalgam /solder
- Reaction Between Xand Y ,X loses electron and Y gains and form Z what are the properties of Z
- Electric configuration Of X is 2,8 Y has 2,8,7 Z has 2,8,2 which of them is metal
- Which reaction is possible MgSO4 +Fe/ ZnSO4 + Fe/ CuSO4 +Fe and why ?
- Which are the constituents of solder which property is used in it to join the wire
- A metal liquid at room temperature can be obtained from it’s sulphide identify the metal and reaction
- what happens when a) ZnCO3 is heated in absence of air b) mixture of Cu2O and Cu2S is heated c) Name metal and non-metal in form of liquid at room temperature. d) what is roasting and calcination of Znic ore.
- An element A react with water to form compound B whichis used in white wash on heating it changes toC which if treated with water gives B again write compound A,B,C and reaction related to it.
- Metal A does give H2 when react with dilute acid but gives black substance B when reacted with oxygen what is A and B
- CuSO4 solution is kept in iron container after few day the containers had many wholes why. write the reaction.
- Explain why a)Reactivity of Al reduces if it is dipped in HNO3 b) Carbon cannot reduce Na and Mg oxides
- Two metal ore A and B taken on heating A gives CO2 and B gives SO2 what steps will you take to covert into metal.
Metals Non Metals and Carbon
Compounds Questions
Related To Year Questions
- A metal and Non metal found in liquid state Ans: Mercury and Bromine(2024)
- (Important Reactions Metals Non Metals and Carbon Compounds Questions )Which reactions are possible a) CaSO4+ Al—-> b) CuSO4+Ca—.> c) FeSO4 +Cu —-> d) ZnSO4 +Mg—->
- i)It is observed that Ca when reacted with water floats on the surface of water why it happens so, write balance equation what happens when CO2 is reacted with the product ii) State electroytic refining of copper.
- Important Question 2025 :The metals obtained from their molten chlorides by the process of electrolytic reduction are :
(A) Gold and silver(B) Calcium and magnesium(C) Aluminium and silver(D) Sodium and iron - The formation of magnesium oxide is correctly shown in option :
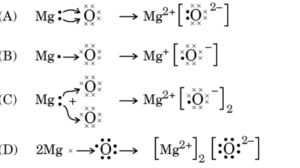
- Tooth enamel is made up of calcium hydroxyapatite (a crystalline form of calcium phosphate). This chemical starts corroding in the mouth when the pH is :(A) 7 (B) 5 (C) 10 (D) 14
- The products formed when Aluminium and Magnesium are burnt in the presence of air respectively are :(A) Al3O4and MgO2
(B) Al2O3and MgO (C) Al3O4 and MgO (D) Al2O3 and MgO2 - Translate the following statements into chemical equations and then balance them : (a) Nitric acid reacts with calcium hydroxide to form calcium nitrate and water.(b) Sodium chloride reacts with silver nitrate to form silver chloride and sodium nitrate.
- With the help of an activity, explain the conditions under which iron articles get rusted. Name two metals which react violently with water. List any three observations which a student notes when these metal are dropped in a beaker containing water. Write a test to identify the gas evolved (if any) during the reaction of these metals with water.
- ‘‘Displacement reactions also play a key role in extracting metals in the middle of the reactivity series.’’ Justify this statement with
two examples. Why can metals high up in the reactivity series not be obtained by reduction of their oxides by carbon ? - Aluminium powder is used in thermit welding because :(A) Its reaction with iron is highly exothermic.(B) When it is heated with iron (III) oxide, molten iron is obtained.(C) When it is heated with iron (III) oxide, molten aluminium oxide is obtained to join railway tracks.(D) Its melting point is low as compared to iron and a molten alloy of iron and aluminium is formed on heating which is used to join railway tracks.
- In common practice silver is recovered from silver nitrate solution by the use of copper metal. Name the type of reaction that takes
place in this process and give the chemical equation of the reaction involved.(b) Name the method used for refining silver - 8. Write the electron-dot structures of (i) sodium, and (ii) oxygen. Using these structures, show the formation of sodium oxide. Mark the anion and cation present in this compound. (At. No. – Sodium = 11 and Oxygen = 8)
- The most common method of extraction of metals from their oxide ores is :(A) Reduction with carbon(B) Reduction with hydrogen
(C) Reduction with aluminium (D) Electrolytic reduction - (i) Consider the following metals :K, Ca, Al, Cu, Ag, Fe Select from the above metals, a metal which
I. does not react with oxygen even at high temperature. II. reacts with oxygen at ordinary temperature and forms a protective oxide layer which prevents the metal from further oxidation. III. catches fire when kept in the open.IV. does not burn in oxygen but the hot metal is coated with a black coloured oxide layer.(ii) What are amphoteric oxides ? With the help of balanced chemical equations show that aluminium oxide is an amphoteric oxide.(iii) What are alkalis ? Give one example. - ) (i) With the help of balanced chemical equations state the process of extracting (I) mercury from its ore called cinnabar,
and (II) copper from its sulphide ore.(ii) Silver and copper articles slowly lose their shiny surfaces when exposed to air. Name the compounds formed on(I) silver articles, and (II) copper articles in the form of coating.
Metals Non Metals and Carbon Compounds Questions Related to
CARBON AND IT’S COMPOUND

- Write the electron dot structure of ethyne C2H2 ,ethanol C2H5OH methane CH4 and ethane C2H6. Draw the structure of ethanol and ethanoic acid . Why is the conversion of ethanol to ethanoic acid considered oxidation reaction write the oxidizing agent
- What is homologous series of carbon compounds . Make the choice i)C2H6O ii)C2H6O2 iii)C2H6 iv)CH4O
- Why is carbon able to form numberless carbon compounds. why does it form covalent compounds
- A compound A (C2H4O2)react with Na to form B compound evolve the gas with pop sound ,if A is treated with alcohol it gives C in the presence of acid to form the sweet smelling compound (C4 H8O2) on addition with NaOH it forms D name the compound A,B,C,D.
- what happens when ethanol is burnt in air. what happens when ethanol is reacted with Na. write it’s structural formula of ethanol what happens when it is heated with excess of conc.H2SO4 at443k.
- Write the properties of co-valent compounds. why are alkanes also called paraffins.
- What is saponification? Write the difference between soaps and detergents ,it’s chemical composition ,action with hard water and biodegradability..
- Give IUPAC name of the following i) CH3-CH2-Br ii) CH3-CH2-CH2-CH2-C=CH2 iii)CH3-CH2-CHO iv) CH3 – CH2 -OH
- Write the physical properties of ethanoic acid and ethanol
- What are isomers ,draw the structure of two isomers of butane pentane?
- What happens when ethene react with water in presence of concH2SO44
- Why the reaction of methane and chlorine is called substitution reaction
- Complete the reaction a)CH3COOH +Na2CO3 ——> b)C2H5OH +Na ———> c)CH4 + Cl2 —– sunlight —-> d) CH3CH2OH +[O] ——alkaline KMnO4—> e)CH2=CH2+H2 —-Ni——> f) C H3-CH2 OH —- conc H2SO4/443k —–> g) CH3COOH + NaHCO3 —–> h) CH3COOH +C2H5OH ——–> i) CH3COOC2H5 +NaOH ——-> j) CH3COOH +NaOH —->
- What is fermentation How is Vinegar produced by ethanol and by methanol write the chemical equation for both the cases write the use of acetic acid
- On dropping a small piece of Na into the organic compound A with molecular formula C2H6O in a test tube a brisk efference is observed with pop sound with bringing the burning splint near the mouth of test tube, A changes to B when heated in excess of conc.H2SO4. write the equation find A and B.
- An Organic compound A molecular formula C2H4O2 react with Na gives gas B which readily catches fire. A react when react with ethanol in the presence of conc H2SO4 to form sweet smelling substance C Find A,B,C. write the chemical equation.
- What happens when an ester is treated with an alkali solution. Hydrolysis of ester
- Two carbon compounds A and B molecular formula C3H8 and C3H6 which of them is likely to have addition reaction write the reaction useful vegetable oil to ghee.
Metals Non Metals and Carbon
Compounds Questions
Related to Year Questions
- What are micelles ?How does it help to clean the clothes
- Carbon Compounds are : Ans bad conductor of electricity and have weak force of attraction between the molecules ? Define the homologues series of carbon compounds ? Why the melting point of C4H8 is higher than C3H6 or C2H4 ? Why do we not see any gradation in chemical properties in homologous series compounds? Write the name and structure of aldehyde and Ketone with molecular formule C3H6O (Metals Non Metals and Carbon Compounds Questions2024)
- Write the name and structure of the organic Compound X having two carbon atoms in its molecule its suffix is ‘ ol’ what happens when X is heated with the conc. H2SO4 at 443k ? Write the equation and state the role of conc. acid Draw the dot structure of X (Metals Non Metals and Carbon Compounds Questions2024) ii) Give the reason that Carbon can neither be C^4+ or C^4- but forms covalent compounds . iii) write the chemical formula of 2 homologous of aldehyde iv) draw the srtucture of cylohexane(Metals Non Metals and Carbon Compounds Questions2024)
- Name the commercially important compound having –OH group write the molecular formula, It’s reaction with a)Sodium b)Excess of conc. H2SO4 c) Ethanoic acid d) acidified K2Cr2O7(Metals Non Metals and Carbon Compounds Questions2024)
- Important Questions 2025:Draw electron dot structure of chlorine molecule. (Atomic Number of Chlorine = 17)What happens when chlorine reacts with methane in the presence of sunlight ? Write the name of the reaction
- Name the two oxidising agents used for the conversion of alcohols to acids.List four differences in properties between covalent
compounds and ionic compounds. - Give reason why carbon forms compounds mainly by covalent bonding.(ii) Why do covalent compounds have low melting and boiling points.(iii) Give reason for the following :I. Covalent compounds are bad conductors of electricity.II. Carbon shows catenation.
- Select from the following the members of same homologous series :
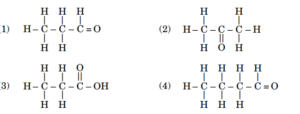
- What happens to (i) the melting point, and (ii) the solubility of compounds as the molecular mass of the compounds in a
homologous series increases ? - (i) Differentiate between saturated and unsaturated hydrocarbons by giving one example each, with a structuralformula.
(ii) Write the method of converting an unsaturated hydrocarbon into a saturated hydrocarbon. Name the industry where this
reaction is commonly used.(iii) Write the name and structure of a hydrocarbon having double bond and four carbon atoms in its one molecule. - Given below are the structures of some hydrocarbons. Select the two
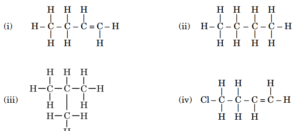 structures which are related to each other from the given options :(A) (i) and (iv) (B) (ii) and (iv) (C) (ii) and (iii) (D) (i) and (iii)
structures which are related to each other from the given options :(A) (i) and (iv) (B) (ii) and (iv) (C) (ii) and (iii) (D) (i) and (iii) - Choose the incorrect statement about the common reaction used in hydrogenation of vegetable oils.
(A) It is an addition reaction.(B) It takes place in the presence of nickel or palladium catalyst.(C) The product contains only single bonds between carbon atoms.(D) It is an addition reaction which occurs in the presence of an acid catalyst. - Design an experimental set-up to demonstrate that ‘‘Alcohol and glucose contain hydrogen but are not categorised as acids’’. Also
give the reason to justify this fact.