Chemical Reaction and Acid Bases and Salts Important Questions
Chemical Reaction and Acid Bases and Salts Important Questions is the unit of Science of Class 10 CBSE consist of Year wise 2018 to 2024 topic and chapter wise questions
CHEMICAL REACTION
- a)*Iron III oxide reacts with Aluminum and gives iron and aluminum oxide. write the balance equation it is also reduced b)dil H2SO4 reacts with Al powder c) dil HCl react with Na2CO3 d)* CO2 passes through lime water. e) NaOH solution is heated with Zn f) dil H2SO4 react with Na2CO3 g)egg shell (CaCO3) react with dil HCl h) copper II oxide react with dil HCl. i)Calcium metal is reacted with water j)Cinnabar (HgS) is heated in presence of air k)* MnO2 is heated with aluminum powder l)The compound obtained on reaction of iron and steam. m) Mercury oxide is heated n) mixture of cuprous oxide and cuprous sulphide is heated
- A reddish brown coloured metal ,used in electric wire when powdered and heated becomes black ,when H2 gas is passed over it it regains it’s original colour on the basis of explanation a) name the metal and black colour substance write the balanced equation
- Identify the types of reactions .a) CH4 +2O2 —-> CO2 + 2H2O
- Pb(NO3)2 + 2KI ——-.> PbI2 + 2 KNO3
- ***CaO +H2O ———> Ca(OH)2 + heat
- *CuSO4 +Zn ———> ZnSO4 + Cu .
- AgNO3 (aq) + NaCl (aq) ———> AgCl (s) + NaNo3 (aq)
- 2KClO3 ———-> 2KCl + 3O2
- CaCO3 +H2O + CO2 ——–>
- HCl +H2O ———>
- BaCl2 + H2SO4 ———>BaSO4 (white ppt)+ 2 HCl
- why the blue colour of copper sulphate disappears when some aluminum power is added to it .
- When calcium oxide is taken in container and water is added to it slowly state two observation made the experiment and write the balanced equation a) kind of sound b) exothermic / endothermic reaction. (2024)
- * when H2 gas is passed over heated copper II oxide , copper and steam is formed write the equation write the substance oxidised and reduced .
- A student dropped a few pieces of marble in dil HCl in the test tube the gas evolved passed through lime water .write the balanced equation .
11 write which of following substances are oxidise / reduced
3MnO2+4Al ——-> 3Mn +2Al2O3 ;
Fe2O3 +3CO ——–> 2Fe + 3CO2
SO2 +2H2S ———–>3S + 2H2O
* MnO2 +4HCl ———> MnCl2 +2H2O+Cl2
12. What is corrosion and rancidity , oxidation and reduction,
13. In the electrolysis of water why the volume of gas collected over one electrode is double that of gas collected over another .name the gases on which electrode are they collected
14**What type of reaction is it write the balanced equation when lead nitrate is reacted with potassium iodide ./ Znic react wit silver nitrate to produce znic nitrate and silver.
15 * Write the balanced equation when solution of Barium chloride and Sodium sulphate in water react to give insoluble barium sulphate and solution of sodium chloride. Name the type of reaction what name is given to insoluble substances.(2024)
16 Decomposition reaction requires energy either in form of heat/ light/electricity write thermal decomposition of CaCO3 ,Ag Cl ,H2O
17. 2ml of NaOH solution is added to a few pieces of Zn granules in a test tube when the content is warmed a gas evolved which is bubbled through soap solution before testing . write the equation named the gas test to detect the gas .What happens the same metal is reacted with strong dil acid.
18. When H2S gas is passed through a blue colour solution of copper sulphate a clack precipitate of copper sulphide is obtained and sulphuric acid is obtained this reaction is rhe example of ————— reaction.
important question 2024
When 2 ml of sodium hydroxide solution is added to few pieces of Znic granulated in a test tube and than warmed the reaction occurs :
Ans : 2NaOH +Zn ——->Na2ZnO2 (sodium Zincate) +H2
The decomposition reaction in which source of energy is light
Ans : 2AgBr ——-sunlight —–> 2Ag + Br2 ( see notes for more examples )
3. Consider the compounds with maximum number of water of crystallisation
FeSo4 , CuSO4 , CaSO4 and Na2SO4
Ans : Washing Soda Na2SO4 . 10 H2O , Green Vitriol FeSO4 .7H2O, blue Vitriol CuSO4 .5H2O, Gypsum CaSO4.2H2O
4.MnO2 +4HCl ———> MnCl2 + 2H2O +Cl2 the reaction given is redox reaction because
Ans: MnO2 is reduced and HCl is oxidized
Important Question (2025)
1.Electrolysis of water is a decomposition reaction. The mass ratio of hydrogen and oxygen gases liberated at the electrodes
during electrolysis of water is :(A) 8 : 1 (B) 2 : 1(C) 1 : 2
2.Reaction between two elements A and B, forms a compound C. A loses electrons and B gains electrons. Which one of the following properties will not be shown by compound C ?(A) It has high melting point.(B) It is highly soluble in water.(C) It has weak electrostatic forces of attraction between its oppositely charged ions.(D) It conducts electricity in its molten state or aqueous solution
3.. A student performs the following experiment in his school laboratoryList two observations to justify that in this experiment a chemical change has taken place. 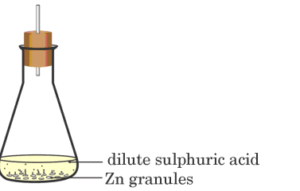
4.The correct balanced chemical equation showing exothermic reaction in which natural gas burns in air is :
(A) CH4 + O2 ⎯⎯→CO2+ 2H2O(B) CH4+ 2O2 ⎯⎯→2CO2 + 2H2O + Energy(C) CH4+ 2O2 ⎯⎯→CO2 + 2H2O
(D) CH4+ 2O2 ⎯⎯→CO2 + 2H2O + Energy
5.Consider the following chemical equation :p Al + q H2O ⎯⎯→r Al2O3+ s H2 .To balance this chemical equation, the values of ‘p’, ‘q’, ‘r’ and ‘s’ must be respectively :(A) 3, 2, 2, 1 (B) 2, 3, 3, 1(C) 2, 3, 1, 3 (D) 3, 1, 2, 2
6.List the possible sources of energy required in decomposition reactions. Illustrate any one with a suitable example
7.Write balanced chemical equation to show the reaction of iron (III) oxide (Fe2O3) with aluminium.
8(i) Define the term decomposition reaction. Write one chemical equation each for decomposition reaction where energy is
supplied in the form of heat, light or electricity.(ii) Decomposition of vegetable matter into compost isconsidered an exothermic reaction. Why ?
9Why are decomposition reactions called the opposite of combination reactions ? Write one chemical equation each for these two types of reactions mentioning the name of the reactant(s) and the product(s) involved in the reactions.
10.Choose the incorrect statement about the common reaction used in hydrogenation of vegetable oils.(A) It is an addition reaction.
(B) It takes place in the presence of nickel or palladium catalyst.(C) The product contains only single bonds between carbon atoms.
(D) It is an addition reaction which occurs in the presence of an acid catalyst.
ACID BASES AND SALT
-
- A solution turns red litmus blue its pH value may be 1,4,5,10
- A solution react with crushed egg shell to give a gas that turns lime water milky. The solution contains HCl or NaCl
- Which medicine is used for the treatment of indigestion.
- What happens when a solution of acid react with the solution of base in a test tube i) salt is formed ii)temperature of solution decreases iii ) temperature of solution rises.
- As aqueous solution turns red litmus blue excess addition of which solution will reverse the change i) baking Soda ii)HCl
- During the preparation of HCl gas why is it passed through CaCl2 .
- Give the formula of Baking Soda, Blue vitriol ,washing soda , Gypsum
- Sodium carbonate is basic salt because it is i)strong acid and weak base ii) weak acid and strong base
- Calcium phosphate is present in tooth enamel is acidic /basic.
- Change of pH paper yellow orange changes to green blue can either be antacid /common salt/vinegar.
- If a few drops of concentrated acid spills over the hand the student wash the hand with the saline or wash the hand of plenty of water and put the paste of NaHCO3.
- Sodium hydrogen carbonate when added to acetic acid evolve gas i) turns lime water milky ii) it extinguishes a burning splinter iii) it dissolves in the solution of NaOH iv) it has a pungent smell select the option.
- One of the constituents of baking powder is NaHCO3 other one is HCL/Tartaric acid
- Which statement is correct a) higher the pH stronger the acid b) higher the pH weaker the acid c) lower the pH stronger the base d) lower the pH weaker the base
- The pH value of gastric juice released during digestion is more than7 or less than 7
- Name the phenomena when small amount of acid is added to water a)ionization and neutralization b)Dilution and Neutralization c)ionization and dilution
- Which of the following can be used as acid -base indicator by visually impaired students a) turmeric b) Vanilla essence c) litmus
- CO2 gas is not produced on the treatment of following with dilute acid a) Marble b) Limestone c)baking soda d) lime (CaO)
- Lime juice/lime water which contain acid.
- By demonstrating the electrical conductivity through electrolyte a) the bulb glows because NaOH is strong base and produce ions for conduction b)blub will not glows as electrolyte is not acid fig
- Onion, clove and Vanilla is a kind of _________ indicators
- Which is correctly matched i)acid +salt ——–Metal +H2 ii)Acid +Metal carbonate ——-> salt +CO2+H2O iii) Metal oxide + acid ——-> Salt +water
- Which is correct a)adding acid to water by stirring b) adding water to acid by stirring.
- Which of the statements are correct for pH scale a)A scale for measuring hydronium ion concentration b)Values less than 7 represent acidic medium c)as pH increases from 7 to 14 it represent increase in hydrogen ion.
- Which statements are correctly matched a)pH of plants and animals lies 7 -7.8 b)pH of rain water is 7.6 c) Tooth decay when pH is 5.5 d) common salt is forrmed by NaOH and HCl e)Brine is aqueous solution of NaCl f)Chlor – alkali process is used for the formation of NaCl.
- Formula of plaster of Paris_______
- Which is responsible of acid rain a)H2S and CO2 b)oxides of Sulphur and nitrogen c) oxides of Sulphur and CO2
- On washing clothes with soap a turmeric stain on cloth turns red because soap is acidic /alkaline
- Assertation : Olfactory indicators are those whose colour changes in acidic medium Reason :They react with acidic and basic solution ANS both are false as olfactory indicators whose colour changes inacdicas wel as basic media .
- Assertation :Active metal react with acid to give hydrogen REASON ;It is the example of displacement reaction ANS; Both are true
- Assertation :Acids contain H+ ion REASON : H+ ion naturalize acid ANS :assertation is true but reason is wrong.
- Assertation : the process of dissolving acid or base in water is highly exothermic Reason :A large amount is produced ANS ; Both are true.
- Assertation: On heating the colour of hydrated copper sulphate changes from blue to white REASON : Copper sulphate is a crystalline salt. ANS Both are correct
- which is HCL or acetic acid is strong acid why?
- Write the equation of a)dissociation of HCl in water b)formation of hydronium ions c) NaOH/KOH dissolved in water.
- Name the acids present in a) Vinegar b)sour milk c) orange d) lemons
- The enamel of tooth is the hardest substances in the body how it gets damage while eating sweets.
- Name the gas produced when metal is reacted with dil. H2SO4 how will you test tge gas evolved.
- Calcium compound which is yellowish white powder usedas disinfectant and also in textile industry. Which gas is released on its preparation name the compound and write the preparation with balanced equation.
- A metal compound react with dil H2SO4 produce the gas which extinguish the burning candle name the gas and write the equation.
- Give only one word for the statements a) water soluble base b) a substance which dissociates on dissolving in water gives hydrogen ions c) a reaction between acid and base to give salt and water d) a substance produce OH- ion on dissolving in water.
- The soil field is highly acidic what minerals should be mixed to reduce the acidity
- What is the effect on litmus paper in aqueous solution of NaCl and NaOH and why ?
- Explain why an aqueous solution of NaSO4 is neutral whereas Na2CO3 is basic.
- Why milkman add baking soda in fresh milk in very small amount why does shit the pH to alkaline and why does this milk take long time to make curd.
- A compound X is used in making Pakoras in kitchen .It is also used to remove indigestion. identify X what happens when heated. name the gas produced and which substance is added to make the cake spongy.
- How is plaster of Paris is prepared why is it stored in moist proof containers.?
- Why happens to anhydrous CuSO4 when few drops of water falls on it ?
- What happens when Zn is treated with NaOH write the equation.?
- What happens when base react with non-metals. ?
- Why do acid do not show acidic character in absence of water .?
- Write the uses of NaOH ,Chlorine which is produced in Chlor – Alkali process name other by products
- how will you prepare washing soda explain with equation and uses
- Illustrate any three chemical properties of acids with examples.
- what happens to blue litmus when added to soda water.?,why common salt becomes sticky during rainy season, ?Why CaCl2 used as desiccator.?
- A tarnish copper vessel shine when rubbed by lemon why? why all alkali are base butall bases are not alkali Why do we apply mild base on the sung of honey bee which acid do they release.
- The pH value of Tomato Juice is 4.6 How it is likely to taste Justify yr answer? How weak and strong acids are different in the ion formation in aqueous solution .? Why is acid rain dangerous for aquatic animals and plants . (2024)
- Important Question 2025 :The following table shows the pH values of four solutions A, B, C and D on a pH scale :The solutions A, B, C and D respectively are of (A) Strong acid, weak acid, neutral, strong base
(B) Weak acid, neutral, weak base, strong base
(C) Weak acid, neutral, strong base, weak base
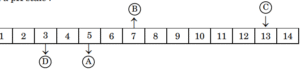
(D) Weak acid, neutral, strong base, strong acid
59.Consider the following reactions :
(i) Dilute hydrochloric acid reacts with sodium hydroxide.(ii) Magnesium oxide reacts with dilute hydrochloric acid.(iii) Carbon dioxide reacts with sodium hydroxide. It is found that in each case :(A) Salt and water is formed.(B) Neutral salts are formed.(C) Hydrogen gas is formed.(D) Acidic salts are formed.
60. In one formula unit of salt ‘X’, seven molecules of water of crystallisation are present. The salt ‘X’ is :(A) CuSO4(B) Na2CO3(C) FeSO4
(D) CaSO4
61.Define water of crystallisation. Give two examples of salts that have water of crystallisation.
62.The warning sign shown in the given figure must invariably be displayed/pasted on the containers which contain hydroxide of :
(A) Aluminium (B) Calcium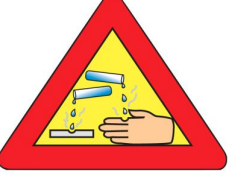 (C) Sodium (D) Magnesium
(C) Sodium (D) Magnesium
63.Which of the given option represents a family of salts ?
(A) NaCl, Na2SO4, CaSO4(B) K2SO4, Na2SO4, CaSO4(C) NaNO3, CaCO3, Na2CO3(D) MgSO4, CuSO4, MgCl2
64.What is observed when hydrated ferrous sulphate crystals are heated in a dry boiling tube ? Give balanced chemical equation(s)of the reactions(s) that occur(s).
65Write the formula of the ions which (i) acids, and (ii) bases generate in water solutions. Dry HCl gas does not change the colour of dry litmus paper. Why ?
66.Common salt is an important raw material for various chemicals of daily use. State in brief the method of preparation of
(i) Sodium hydroxide, and (ii) Sodium hydrogen carbonate from common salt. Write balanced chemical equations of the reactions
that occur.
67.(i) A compound ‘X’ having two carbon atoms in its molecule turns blue litmus red and 5 – 8% solution of ‘X’ in water is
widely used as a preservative. Identify the compound ‘X’ and write its structure. (ii) Compare its pH nature with a mineral acid.
(iii) ‘X’ on reacting with alcohols produces sweet smelling compounds, used in making perfumes. Name the reaction
and write its chemical equation.(iv) When sodium carbonate is added to ‘X’, a colourless gasnis produced which turns lime water milky. Write the chemical equation for the reaction giving the name of the salt produced